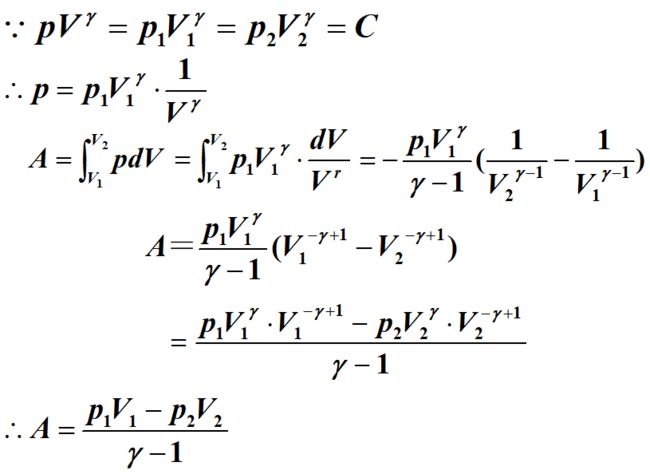热力学第一定律 等值过程 绝热过程
热力学第一定律
内能,功和热量
-
实际气体内能:所有热分子热运动的动能和分子势能的总和
-
内能是状态量: E = E ( T , V ) E=E(T,V) E=E(T,V)
理想气体内能: E = M M m o l i 2 R T E={\frac{M}{M_{mol}}{\frac{i}{2}}RT} E=MmolM2iRT
是状态参量T的单值函数
-
系统内能改变的两种方式
- 做工可以改变系统的状态:摩擦升温(机械功),电加热(电功)
- 热量的传递可以改变系统的内能:热量是过程量
准静态过程
热 力 学 过 程 = { 准 静 态 过 程 非 静 态 过 程 热力学过程 = \left\{ \begin{array}{lr} 准静态过程\\ 非静态过程 \end{array} \right. 热力学过程={准静态过程非静态过程
- 准静态过程:系统从一个平衡态到另一个平衡态,如果过程中所有的中间态都可以近似的看作平衡态法过程
- 准静态过程是理想化过程

弛豫时间 τ \tau τ: 系统从一个平衡态变道相邻平衡态所经过的时间
当 Δ t 过 程 > > τ \Delta t_{过程}>>\tau Δt过程>>τ: 过程就可以视为准静态过程,故无限缓慢只是一个相对的概念。
非静态过程: 系统从一平衡态到另一平衡态,过程中所有中间态为非静态的过程
- 准静态过程曲线
p-V图上,一个点代表一个平衡态,一条连续的曲线代表一个准静态过程
准静态过程的功与热
体积功:
当活塞移动微小位移 d l dl dl时,系统外界所做的元功为:
d A = F d l = p S d l = p d V dA = Fdl = pSdl = pdV dA=Fdl=pSdl=pdV
A = ∫ V 1 V 2 p d V A=\begin{aligned} \int_{V_{1}}^{V_{2}} p \mathrm{d} V \end{aligned} A=∫V1V2pdV
d V > 0 , d A > 0 dV>0,dA>0 dV>0,dA>0系统对外界做正功
d V < 0 , d A < 0 dV<0,dA<0 dV<0,dA<0系统对外界做负功
d V = 0 , d A = 0 dV=0,dA=0 dV=0,dA=0系统不做功
- 功是过程量
- 做功改变系统热力学状态的微观实质
-功是系统与外界交换的能量的量度
准静态过程中的热量计算
C = d Q d T C = \frac{dQ}{dT} C=dTdQ
C(热容量):系统在某一无限小过程中吸收热量 d Q dQ dQ与温度变化 d T dT dT的比值
单位: J ⋅ K − 1 J\cdot K^{-1} J⋅K−1
热容量与比热的关系为: C = M c 比 C = Mc_{比} C=Mc比
C m C_m Cm(摩尔热容量):
C = M M m o l C m C = {\frac{M}{M_{mol}}}{C_{m}} C=MmolMCm
d Q = M M m o l C m d T dQ = {\frac{M}{M_{mol}}}{C_m}{dT} dQ=MmolMCmdT
Q = M M m o l C m ( T 2 − T 1 ) Q = {\frac{M}{M_{mol}}}{C_m}(T_2-T_1) Q=MmolMCm(T2−T1)
- 传热的微观本质:
- 热量也是能量变化的量度
热力学第一定律
对于任一过程,系统与外界可能同时有功和热量的转换,且系统能量改变仅为内能时,根据能量守恒:
Δ E = Q + ( − A ) \Delta E= Q + (-A) ΔE=Q+(−A)
或 Q = Δ E + A Q = \Delta E + A Q=ΔE+A
-
Q > 0 Q>0 Q>0系统吸热, Q < 0 Q<0 Q<0系统放热
-
A > 0 A>0 A>0系统对外做功, A < 0 A<0 A<0外界对系统做功
-
Δ E > 0 \Delta E> 0 ΔE>0系统内能增加, Δ E < 0 \Delta E<0 ΔE<0系统内能减少
-
如果系统经历一些微小变化过程,则 d Q = d E + d A dQ=dE+dA dQ=dE+dA;
-
对准静态过程:
d Q = d E + p d V dQ=dE+pdV dQ=dE+pdV
Q = Δ E + ∫ V 1 V 2 p d V Q=\Delta E + {\begin{aligned}{\int_{V_{1}}^{V_{2}}}p{\mathrm{d} V}\end{aligned}} Q=ΔE+∫V1V2pdV
理想气体等值过程
等容过程,定容摩尔热容
∵ d V = 0 , d A = p d V = 0 \because dV=0,dA= pdV = 0 ∵dV=0,dA=pdV=0
∴ d Q = d E = M M m o l i 2 R d T \therefore dQ=dE={\frac{M}{M_{mol}}}{\frac{i}{2}}RdT ∴dQ=dE=MmolM2iRdT
Q V = E 2 − E 1 = M M m o l i 2 R d ( T 2 − T 1 ) Q_V=E_2-E_1={\frac{M}{M_{mol}}}{\frac{i}{2}}Rd(T_2-T_1) QV=E2−E1=MmolM2iRd(T2−T1)
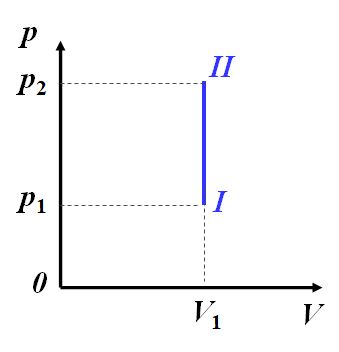
定容摩尔热容量
d Q V = d E = i 2 R d T dQ_V=dE={\frac{i}{2}}RdT dQV=dE=2iRdT
C V = ( d Q d T ) V C_V=({\frac {dQ}{dT}})_V CV=(dTdQ)V
C V , m = i 2 R C_{V,m}={\frac{i}{2}}R CV,m=2iR
- 单原子理想气体: C V , m = 3 2 R C_{V,m}={\frac{3}{2}}R CV,m=23R
- 双原子理想气体: C V , m = 5 2 R C_{V,m}={\frac{5}{2}}R CV,m=25R
- 多原子理想气体: C V , m = 3 R C_{V,m}=3R CV,m=3R
理想气体内能
E = M M m o l C V , m T E={\frac{M}{M_{mol}}}{C_{V,m}}T E=MmolMCV,mT
理想气体的任一 T 1 → T 2 T_1\rightarrow T_2 T1→T2过程
d E = ν C V , m d T dE=\nu C_{V,m}dT dE=νCV,mdT
Δ E = E 2 − E 1 = ν ∫ T 1 T 2 C V , m d T \Delta E=E_2-E_1={\nu}{\begin{aligned}{\int_{T_{1}}^{T_{2}}}{C_{V,m}}{\mathrm{d} T}\end{aligned}} ΔE=E2−E1=ν∫T1T2CV,mdT
若 C V , m C_{V,m} CV,m近似为常数,则有 Δ E = ν C V , m Δ T \Delta E = \nu C_{V,m}\Delta T ΔE=νCV,mΔT
等压过程,定压摩尔热容
d A = p d V dA=pdV dA=pdV
d Q p = d E + p d V dQ_p=dE+pdV dQp=dE+pdV
A p = ∫ V 1 V 2 p d V = p ( V 2 − V 1 ) A_p={\begin{aligned}{\int_{V_{1}}^{V_{2}}}p{\mathrm{d} V}\end{aligned}}=p(V_2-V_1) Ap=∫V1V2pdV=p(V2−V1)
Q p = M M m o l i 2 R ( T 2 − T 1 ) + M M m o l R ( T 2 − T 1 ) Q_p={\frac{M}{M_{mol}}}{\frac{i}{2}}R(T_2-T_1)+{\frac{M}{M_{mol}}}R(T_2-T_1) Qp=MmolM2iR(T2−T1)+MmolMR(T2−T1)

定压摩尔热容量
d Q p = d E + d A p = C V , m d T + p d V dQ_p=dE+dA_p=C_{V,m}dT+pdV dQp=dE+dAp=CV,mdT+pdV
p V = R T 微 分 得 p d V = R d T pV=RT微分得pdV=RdT pV=RT微分得pdV=RdT
d Q p = i 2 R ⋅ d T + R ⋅ d T dQ_p={\frac{i}{2}}R\cdot dT+R\cdot dT dQp=2iR⋅dT+R⋅dT
C p , m = ( d Q d T ) p C_{p,m}=(\frac{dQ}{dT})_p Cp,m=(dTdQ)p
C p , m = i 2 R + R C_{p,m}={\frac{i}{2}}R+R Cp,m=2iR+R
C p , m = C V , m + R C_{p,m}=C_{V,m}+R Cp,m=CV,m+R
Q p , m = M M m o l C p , m ( T 2 − T 1 ) Q_{p,m}={\frac{M}{M_{mol}}}{C_{p,m}}(T_2-T_1) Qp,m=MmolMCp,m(T2−T1)
比热容比: γ = C p , m C V , m \gamma =\frac{C_{p,m}}{C_{V,m}} γ=CV,mCp,m为绝热系数
理想气体: γ = C p , m C V , m = i 2 R + R i 2 R = i + 2 i \gamma =\frac{C_{p,m}}{C_{V,m}}=\frac{{\frac{i}{2}}R+R}{{\frac{i}{2}}R}=\frac{i+2}{i} γ=CV,mCp,m=2iR2iR+R=ii+2
- 对单原子分子: i = 3 , γ = 1.67 i=3,\gamma=1.67 i=3,γ=1.67
- 对刚性双原子分子: i = 5 , γ = 1.40 i=5,\gamma=1.40 i=5,γ=1.40
- 对刚性多原子分子: i = 6 , γ = 1.33 i=6,\gamma=1.33 i=6,γ=1.33
等温过程
d T = 0 , d E = 0 dT=0,dE=0 dT=0,dE=0
d Q T = d A T dQ_T=dA_T dQT=dAT
d Q T = p d V , p = ν R T ⋅ 1 V dQ_T=pdV,p=\nu RT\cdot \frac{1}{V} dQT=pdV,p=νRT⋅V1
Q T = A T = ∫ V 1 V 2 ν R T d V V = ν R T l n V 2 V 1 = p 1 V 1 l n V 2 V 1 Q_T=A_T={\begin{aligned}{\int_{V_{1}}^{V_{2}}}\nu RT{\frac{dV}{V}}\end{aligned}}=\nu RTln{\frac{V_2}{V_1}}=p_1 V_1 ln{\frac{V_2}{V_1}} QT=AT=∫V1V2νRTVdV=νRTlnV1V2=p1V1lnV1V2
⇒ Q T = { p 1 V 1 l n p 1 p 2 = p 2 V 2 l n p 1 p 2 M M m o l R T l n p 1 p 2 \Rightarrow Q_T = \left\{\begin{array}{lr}p_1 V_1 ln{\frac{p_1}{p_2}}=p_2 V_2 ln{\frac{p_1}{p_2}}\\\frac{M}{M_{mol}}RTln{\frac{p_1}{p_2}}\end{array}\right. ⇒QT={p1V1lnp2p1=p2V2lnp2p1MmolMRTlnp2p1
绝热过程
系统变化过程中,系统与外界没有热交换
- 特征: d Q = 0 , d E + d A = 0 dQ=0,dE+dA=0 dQ=0,dE+dA=0
绝热方程
-
对于准静态方程
ν C V , m d T + p d V = 0 \nu C_{V,m}dT+pdV=0 νCV,mdT+pdV=0p V = ν R T pV=\nu RT pV=νRT
取微分得
p d V + V d p = ν R d T pdV+Vdp=\nu RdT pdV+Vdp=νRdT
消去 ν d T \nu dT νdT得
p d V + V d p = − R p d V C V , m pdV+Vdp=-R{\frac{pdV}{C_{V,m}}} pdV+Vdp=−RCV,mpdV
C V , m p d V + C V , m V d p = − R p d V {C_{V,m}}pdV+{C_{V,m}}Vdp=-RpdV CV,mpdV+CV,mVdp=−RpdV
C p , m p d V + C V , m V d p = 0 {C_{p,m}}pdV+{C_{V,m}}Vdp=0 Cp,mpdV+CV,mVdp=0
d p p + γ d V V = 0 {\frac{dp}{p}}+\gamma {\frac{dV}{V}}=0 pdp+γVdV=0
积分得
∫ d p p + ∫ γ d V V = 0 {\begin{aligned}\int \frac{dp}{p}\end{aligned}}+{\begin{aligned}\int \gamma \frac{dV}{V}\end{aligned}}=0 ∫pdp+∫γVdV=0
得
l n p + γ l n V = C lnp+\gamma lnV=C lnp+γlnV=C
l n p V γ = C lnpV^\gamma=C lnpVγ=C
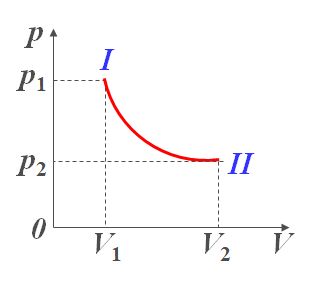
p V γ = C 1 pV^\gamma=C_1 pVγ=C1
p V γ − 1 = C 2 pV^{\gamma-1}=C_2 pVγ−1=C2
p γ − 1 T − γ = C 3 p^{\gamma-1}T^{-\gamma}=C_3 pγ−1T−γ=C3,即松柏方程
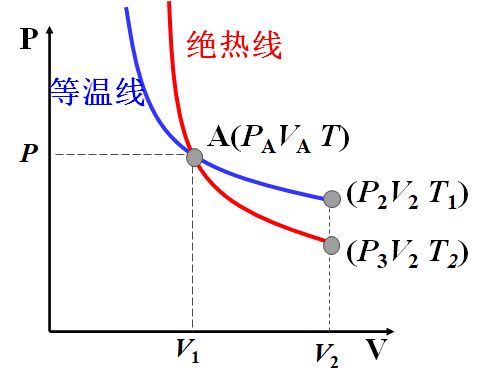
绝热线与等温线
p V = C 1 , 等 温 线 pV=C_1,等温线 pV=C1,等温线
p V γ = C 2 , 绝 热 线 pV^\gamma=C_2,绝热线 pVγ=C2,绝热线
-
对于等温过程
p V = C 1 = p A V A pV=C_1=p_A V_A pV=C1=pAVA
p = C 1 V p=\frac{C_1}{V} p=VC1
d p d V ∣ A T = − C 1 V 2 ∣ A = − C 1 V A 2 = − p A V A V A 2 = − p A V A \frac{dp}{dV}|_{AT}=-\frac{C_1}{V^2}|_A=-\frac{C_1}{V_A ^2}=-\frac{p_AV_A}{V_A ^2}=-\frac{p_A}{V_A} dVdp∣AT=−V2C1∣A=−VA2C1=−VA2pAVA=−VApA
-
对于绝热过程
p V γ = C 2 = p A V A γ pV^\gamma=C_2=p_AV_A ^\gamma pVγ=C2=pAVAγ
p = C 2 V γ p=\frac{C_2}{V^\gamma} p=VγC2
d p d V ∣ A γ = − γ C 2 V γ + 1 ∣ A = − γ p A V A γ V A γ + 1 = − γ p A V A \frac{dp}{dV}|_{A\gamma}=-\gamma \frac{C_2}{V^{\gamma+1}}|_A=-\gamma \frac{p_AV_A ^\gamma}{V_A ^{\gamma+1}}=-\gamma \frac{p_A}{V_A} dVdp∣Aγ=−γVγ+1C2∣A=−γVAγ+1pAVAγ=−γVApA
∵ γ > 1 \because \gamma > 1 ∵γ>1
∴ ∣ d p d V ∣ A γ = γ p A V A > ∣ d p d V ∣ A T = p A V A \therefore |\frac{dp}{dV}|_{A\gamma}=\gamma \frac{p_A}{V_A}>|\frac{dp}{dV}|_{AT}=\frac{p_A}{V_A} ∴∣dVdp∣Aγ=γVApA>∣dVdp∣AT=VApA
即绝热线要陡一些
p = n k T p=nkT p=nkT