新冠患者样本单细胞测序文献汇总
科研工作者的信仰就是将真相大白于天下
NGS系列文章包括NGS基础、转录组分析 (Nature重磅综述|关于RNA-seq你想知道的全在这)、ChIP-seq分析 (ChIP-seq基本分析流程)、单细胞测序分析 (重磅综述:三万字长文读懂单细胞RNA测序分析的最佳实践教程 (原理、代码和评述))、DNA甲基化分析、重测序分析、GEO数据挖掘(典型医学设计实验GEO数据分析 (step-by-step) - Limma差异分析、火山图、功能富集)等内容。
其实有关新冠患者样本的单细胞文献并不太多,bug也是比较明显的,比如入组的临床指征的判断,高血压等伴随疾病的影响以及年龄本身导致的免疫差异,筛选条件的不同对最后的结果影响太大,得出相反结论也感觉正常,还有就是用药,,,取样时间。多个已知变量和未知变量的叠加对揭示真相产生重大挑战,,,可能除了限制可控条件外,只能通过加大样本量进行真相的阐释。提到真相。。。忽然,想起了名侦探柯南里毛利小五郎的一句话:“侦探的信仰就是将真相大白于天下”,the same to great scientists 。
本期对查到的新冠患者样本单细胞测序文献进行汇总,尤其是第二篇!!!!,我想起了相声界的一句话,叫“无人不宗马”,第二篇文献虽然还在预印本,但应该是新冠单细胞文献的扛鼎之作,嘻嘻,不说废话了!
1
Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19
2020年3月15日,四川省人民医院研究团队在medRixv预印本上发表题为Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19的研究内容,除了揭示了免疫细胞比例的变化外,还发现interferon-MAPK途径和TCR/BCR在病毒抵御中的重要作用。
Abstract :The coronavirus disease 2019 (COVID-19) outbreak is an ongoing global health emergence, but the pathogenesis remains unclear. We revealed blood cell immune response profiles using 5’ mRNA, TCR and BCR V(D)J transcriptome analysis with single-cell resolution. Data from 134,620 PBMCs and 83,387 TCR and 12,601 BCR clones was obtained, and 56 blood cell subtypes and 23 new cell marker genes were identified from 16 participants. The number of specific subtypes of immune cells changed significantly when compared patients with controls. Activation of the interferon-MAPK pathway is the major defense mechanism, but MAPK transcription signaling is inhibited in cured patients. TCR and BCR V(D)J recombination is highly diverse in generating different antibodies against SARS-CoV-2. Therefore, the interferon-MAPK pathway and TCR- and BCR-produced antibodies play important roles in the COVID-19 immune response. Immune deficiency or immune over-response may result in the condition of patients with COVID-19 becoming critical or severe.
????滑动查看????
研究背景
(1)新冠疫情严重,致病机制不清楚;
(2)适应性免疫对抵抗病毒感染十分重要,TCR和BCR有利于揭示免疫组库的多样性;
研究方案
sample:PBMC(外周血单核细胞),一共16位参与者,其中1例危重症(critical case,patient 1,male),1例重症(severe case,patient 2),6例轻症(moderate cases,patients 3-8),2例初愈患者(病毒检测阴性)(cured patients, (patient 9-10),3例正常人(normal controls,NC 1-3),1例流感患者(patient 11),1例急性咽炎(patient 12),1例脑梗塞(patient 13)作为对照。
测序数据分析介绍:
工具:Chromium Single Cell 5′ Library and Gel Bead Kit(10X Genomics);Chromium Single Cell V(D)J Reagent Kits
比对:
转录组:GRCh38;
TCR/BCR data :cellranger vdj with–reference = refdata-cellranger-vdj-GRCh38-alts-ensembl-2.0.0 to assemble TCR/BCR chains and determine clonotypes
降维聚类:Seurat v3标准流程;
筛选高变基因:p value < = 0.05 and more than 2 times differential expression range to screen the differential genes
伪时序分析:Monocle 2 (version 2.4.0)
结果分析
细胞分群
作者从16个样本中共获得134620个PBMC细胞,83387个TCR克隆,12601个BCR克隆,其中共有17个细胞类型和56种细胞亚型(Fig 1),包括CD4+ T细胞(LTB,IL7R),CD8+ T细胞(LEF1,CD8A),CD4 cytotoxic T细胞(CST7,CCL4)等。这表明细胞在抵御病毒感染时进化为高分化的细胞亚型,并通过伪时序发现56种细胞亚型共出现3种主要状态(Fig 1E),显示了处于分化期的PBMC的发育轨迹。
Fig.1 Cellular composition of the PBMCs in COVID-19 patients and controls.
(A) Schematic of single-cell immune transcriptome profiles of the PBMCs in COVID-19 patients and normal controls. The PBMCs were isolated for constructing single cell 5‘mRNA, TCR, and BCR libraries using chromium single cell V(D)J v1.1 reagent kits of 10x genomics chemistry.
(B) Integration analysis results of COVID-19 patients and normal controls showing principle component (PC), t-SNE algorithm, and UMAP algorithm visualization. In total, 56 cell subtypes were identified.
(C) Seventeen different cell types. From these types, the 56 cell subtypes were derived.
(D) Cell subtypes in each main cell type (only cell types with two or more subtypes are displayed).
(E) Cell differentiation trajectory analysis, which indicates three states of cells.
????滑动查看????
细胞比例变化
作者比较了危重、重症和轻症与健康对照中细胞比例的变化,其中COVID-19患者中CD1C+_B dendritic cells,CD8 cytotoxic T cells和plasmacytoid dendritic cells(pDC)增加,而B cells和CD4+ T细胞减少(Fig.2A)。然后作者细致的描述了56中细胞亚群中在不同病程下的变化情况,发现在危重症中有许多的细胞亚群(40/56)都会减少,一些细胞亚群如CD8+ T cell和CD4+ T cell以及pDC均已经消失。在重症中多数细胞亚群(45/56)都会增加,表明免疫细胞的过度激活。轻症患者的细胞亚群变化在重症与危重症之间。
Fig. 2 The interferon-MAPK pathway is the key response in PBMCs for SARS-CoV-2 infection.
(A) Comparisons of cell type behaviors of patients with COVID-19 and normal controls.
(B) Cell subtypes and the number of pathways significantly differed between patients with COVID-19 and the normal controls.
(C-F) The enrichment pathways of differential expressed (DE) genes in each cell subtype. (C) The critical patient (patient 1) vs normal controls. (D) The severe patient (patient 2) vs normal controls. (E) The moderate patients (patients 3-6) vs normal controls. (F) The cured patients (patient 9 and 10) vs normal controls.
(G-J) Log2 fold changes of interferon pathway genes in critical, severe, moderate, and cured patients with COVID-19 vs normal controls, respectively. Each point represents a different cell subtype.
(K-N) Log2 fold changes of MAPK pathway genes in critical, severe, moderate, and cured patients with COVID-19 vs normal controls, respectively. Each point represents a different cell subtype.
????滑动查看????
不同细胞亚群中的基因表达变化
作者对每个亚群中COVID-19和对照组进行差异表达分析,并对DE genes进行local string network analysis(Fig.2B-F)。富集的信号通路主要涉及viral mRNA translation,interaction alpha/beta signaling,a mitogen-activated protein kinase(MAPK) pathway,immunology interaction between lymphoid and non-lymphoid cells,MHC class II protein complex,表明靶向病毒的抗原提呈已经激活。在重症患者中,超过一半细胞亚群中18条信号通路显著变化,最为显著的是干扰素相关信号通路的过度激活,在其他的COVID-19病程中也发现了该通路不同程度的激活。为了判定18条信号通路是否仅在COVID-19患者中有显著变化,作者将COVID-19病人与流感患者、急性咽炎患者和脑梗塞患者进行比较,经过筛选发现结果保持一致。在这边通路中,MAPK信号通路是抵抗COVID-19的主要的血液免疫反应。
干扰素激活MAPK信号传导途径涉及一系列防御机制,以对抗病毒感染,随后比较不同状态下的COVID-19患者与对照组,进一步探索其中不同细胞的基因表达差异,研究发现interferon α-inducible protein 27(IFI27)在多种细胞类型中的表达水平增高,包括干扰素相关基因如IFITM1、IFITM3和IFITM6等也在不同种细胞亚群中增高。而在干扰素信号通路下游,作者也发现MAPK信号通路转录因子的表达增加,包括FOS、JUN、JUNB和DUSP1等,它们在治愈患者中则为低表达,该结果表明MAPK信号通路下游可以作为患者恢复的指征(Fig.2K-N)。
TCR和BCR的变化
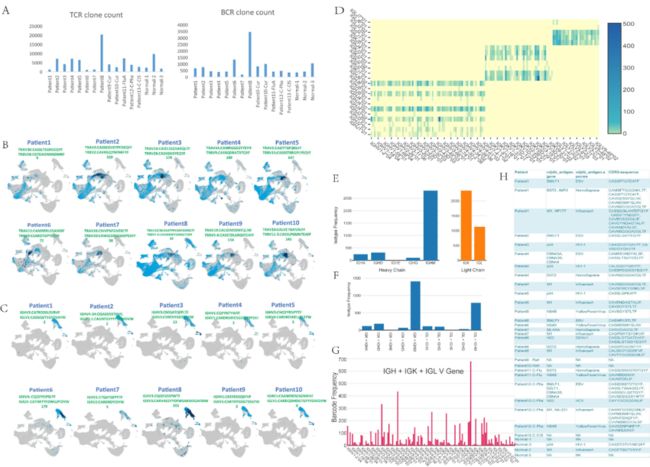
为了探究TCR和BCR的抗体克隆扩增情况,研究团队进行了TCR和BCR V(D)J单细胞转录组分析。在整合分析中,作者发现了83387个TCR细胞克隆和12601个BCR克隆,并且克隆多样性在个体中变化巨大(Fig.3A)。在危重症患者中,TCR克隆数最少,在轻症中,TCR克隆数最多,因此TCR克隆扩增越少,病情越重。
在对top TCR antibody序列进行进化分析时,发现不同患者间的部分TCRa链、CDR3 IGLorK/IGH具有一定的相关性,可能对COVID-19具有特异性。在分析TCR编码已知抗原抗体序列时,在重症患者发现自身抗原反应的明显激活,在危重症中却发现只有拮抗Ebola EBV(Epstein-Barr virus,EBV)抗原的两个抗体的激活,表明危重症情况下的免疫效应不完整,而重症情况下自身免疫的过度放大(Fig3C-H)。
Fig.3 TCR and BCR V(D)J clone expression in patients with COVID-19.
(A) The figure on the left shows the number of TCR clones detected by V(D)J in each patient, and the figure on the right shows the number of BCR clones detected by V(D)J in each patient.
(B) The distribution of TCR clones in cell clusters of patients with COVID-19. The light blue dots indicate the distribution of all TRAV and TRBV clones, and the dark blue dots indicate the antibody sequence and quantity of the clone with the strongest TCR clone signal of the patient 8 (moderate condition).
(C) The distribution of BCR clones in the cell clusters of each patient. Among them, light blue dots indicate the distribution of total IGHV, IGLV, and IGKV in this patient. The dark blue dots indicate the clones with the strongest signals in this patient. Patient 9 has the strongest B cell antibodies among all COVID-19 patients.
(D) V-J heatmap of IGH + IGK + IGL in the B cells of patient 9.
(E) The left and right graphs represent the isotype frequency of the heavy chain and the light chain detected in the B cells of patient 9, respectively.
(F) The distribution of paired isotypes in the B cells of patient \9. Abscissa includes different chains, and the ordinate is frequency.
(G) The usage of the V gene in the B cells of patient 9.
(H) List of the known antigens and antibodies in patients with COVID-19.
????滑动查看????
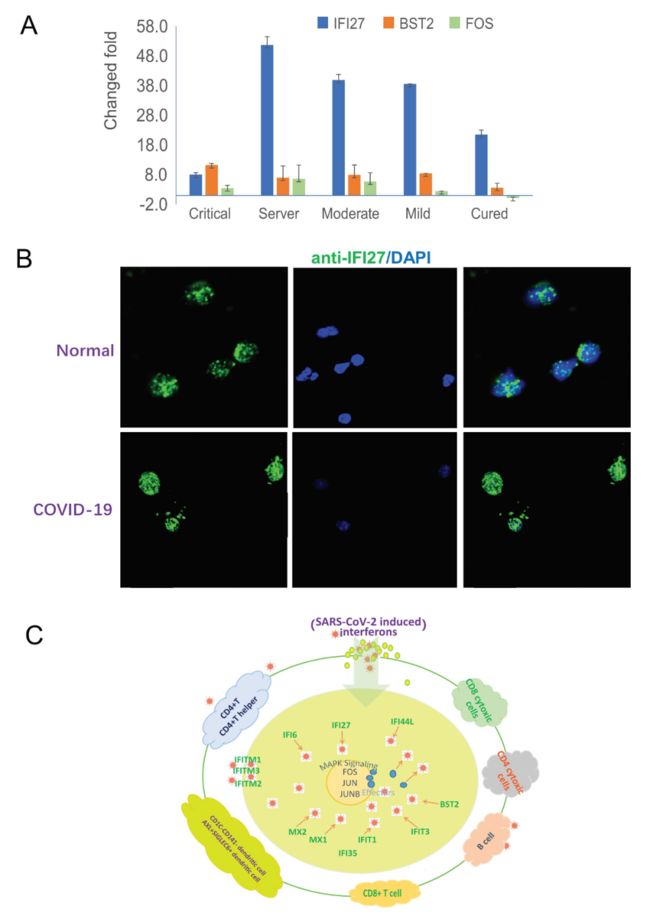
作者对以上观点进行实验验证,并画了模拟图(Fig.4)。作者发现IFI27在新冠肺炎的患者中的表达水平均比正常对照高,表明IFI27可以作为病毒感染的候选marker(我亲爱的authors,你们在这里一个基因用了3个名字,IRF27,IFI27,IIF27,其中IRF27,IIF27都不存在。。。。。),并且作者认为FOS可以成为COVID-19治愈的一个候选marker.在模拟图中,作者说明病毒到达血液免疫细胞后,它可以激活干扰素信号途径的多种细胞亚型,以产生效应因子(例如IFI27,IFITM1和IFITM3)来对抗病毒。通过MAPK,下游效应因子的表达被关键的转录因子FOS、JUN和JUNB被激活,从而导致血液系统中广泛的抗病毒反应。
Fig. 4 The interferon-MAPK pathway in response to SARS-CoV-2 infection.
(A) Real-time PCR validation of IFI27 and BST2 in the interferon pathway and FOS in the MAPK pathway. IFI27 and BST2 are up-regulated in patients with COVID-19. FOS is up-regulated in hospitalized patients but down-regulated in cured patients.
(B) Immunofluorescence staining of IFI27 in PBMCs of patients with COVID-19 and normal controls.
(C) Anti-SARS-CoV-2 response of the blood system. After the virus reaches the blood immune cells, it can activate multiple cell subtypes of the interferon signal pathway to produce effectors, such as IFI27, IFITM1, and IFITM3, to fight the virus. Through downstream activation of MAPK, the expression of downstream effectors is activated by key transcription factors, FOS, JUN, and JUNB, which lead to a wide range of antiviral responses in the blood system.
????滑动查看????
02
A single-cell atlas of the peripheral immune response to severe COVID-19
2020年4月17日,来自斯坦福大学的Catherine团队于medRixv预印本上发表题为A single-cell atlas of the peripheral immune response to severe COVID-19的研究内容,揭示新冠患者PBMC中的表型重构,并提供了针对重症COVID-19免疫反应的细胞图谱。
Abstract:There is an urgent need to better understand the pathophysiology of Coronavirus disease 2019 (COVID-19), the global pandemic caused by SARS-CoV-2. Here, we apply single-cell RNA sequencing (scRNA-seq) to peripheral blood mononuclear cells (PBMCs) of 7 patients hospitalized with confirmed COVID-19 and 6 healthy controls. We identify substantial reconfiguration of peripheral immune cell phenotype in COVID-19, including a heterogeneous interferon-stimulated gene (ISG) signature, HLA class II downregulation, and a novel B cell-derived granulocyte population appearing in patients with acute respiratory failure requiring mechanical ventilation. Importantly, peripheral monocytes and lymphocytes do not express substantial amounts of pro-inflammatory cytokines, suggesting that circulating leukocytes do not significantly contribute to the potential COVID-19 cytokine storm. Collectively, we provide the most thorough cell atlas to date of the peripheral immune response to severe COVID-19.
????滑动查看????
研究背景
(1)重症COVID-19与白介素(IL)-6、IL-10和肿瘤坏死因子(TNF)-α4,5的水平升高有关。尚不清楚在重症患者中较高的炎症水平是否反映了对疾病的适当反应,或者这些反应是否反映了免疫反应失调(通常被称为“细胞因子风暴”);
(2)在严重的COVID-19中经常观察到淋巴细胞减少,特别是CD4 +和CD8 + T细胞减少,而其余T细胞的功能性较低,并且呈现耗竭表型;
研究方案
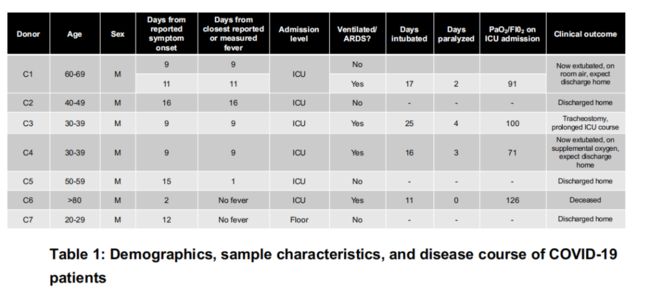
sample:8 peripheral blood samples from 7 hospitalized patients and 6 healthy controls.
测序数据分析介绍:
工具:Seq-Well platform for scRNA-seq
比对: GRCh37 (hg19)
筛选:Cells that had fewer than 1,000 UMIs or greater than 15,000 UMIs, as well as cells that contained greater than 20% of reads from mitochondrial genes or rRNA genes (RNA18S5 or RNA28S5), were considered low quality and removed from further analysis. To remove putative multiplets (where more than one cell may have loaded into a given well on an array), cells that expressed more than 75 genes per 100 UMIs were also filtered out. Genes that were expressed in fewer than 10 cells were removed from the final count matrix.
降维聚类:Seurat
细胞注释:手标+SingleR
基因通路及调控因子分析:Ingenuity Pathway Analysis (IPA; Qiagen)
结果分析
单细胞转录组谱分析捕获SARS-CoV-2感染中外周免疫细胞组成的变化
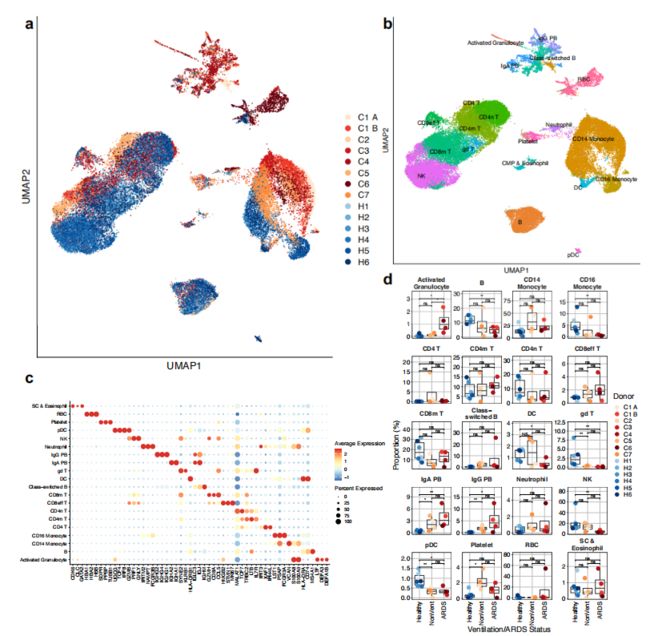
作者一共捕获了44,271个细胞,其中每个样本平均3,194个细胞,并通过UMAP对共30个clusters进行表示(Fig. 1a)。计算亚群的高变基因后通过特异性marker对细胞类型进行标记,并通过SingleR对细胞分型进行确定(Fig. 1b,c)。
在观察细胞比例变化时,发现COVID-19患者的一些先天免疫细胞亚群被耗竭,包括γδT细胞、浆细胞样树突细胞(pDC)、经典树突细胞(DC)、CD16 +单核细胞和NK细胞(Fig. 1d)。并且作者注意到COVID-19患者的浆细胞比例的增加,尤其是在急性呼吸窘迫综合征(acute respiratory distress syndrome,ARDS)患者中较为显著,这表明重症与强烈体液免疫之间的关系。作者在ARDS患者中捕获到了“Activated Granulocytes”,特异性表达中性粒细胞颗粒蛋白的基因如ELANE、 LTF和MMP8。
Figure 1 | Expansion of plasmablasts and depletion of multiple innate immune cell subsets in the periphery of patients with COVID-19.
a, UMAP dimensionality reduction embedding of PBMCs from all profiled samples (n = 44,721 cells) colored by donor of origin. COVID-19 patient IDs (n = 7) begin with “C” and are colored in shades of orange (patients who were not ventilated at time of draw) or red (patients with ARDS who were ventilated at time of draw); healthy donors begin with “H” (n = 6) and are colored in blues.
b, UMAP embedding of the entire dataset colored by orthogonally generated clusters labeled by manual cell type annotation confirmed by SingleR14.
c, Dot plot showing average and percent expression of the 3 most defining genes of each cell type.
d, Proportions of each cell type in each sample colored by peripheral blood of healthy patients (Fig. 1d), the presence of plasmablasts in every COVID-19 sample analyzed suggests evidence of a SARS-CoV-2 humoral immune response. As plasmablasts express high levels of Ig-encoding mRNAs, we examined if we could detect conserved usage of V gene segments in the plasmablasts of COVID-19 patients. Peripheral plasmablasts from COVID-19 patients did not appear to converge on particular Ig V genes
????滑动查看????
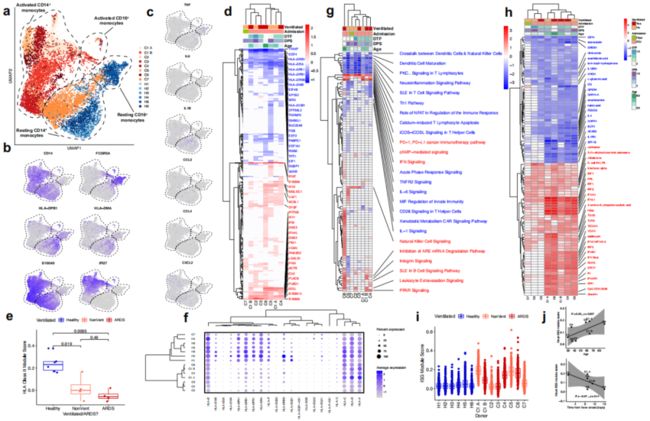
CD14 +单核细胞显示MHC II类下调和IFN驱动的表型重构
在对单核细胞单独进行UMAP降维可视化时发现CD14+ 有强烈的表型偏移和部分CD16+ 细胞的耗竭,在最年轻、病情最轻的患者C7中最不明显(Fig.2a,b)。有趣的是研究者并没有观察到已经报道的外周单核细胞产生的部分促炎因子如TNF、IL6、 IL1B、CCL3、 CCL4、CXCL2(Fig.2c)。这表明循环单核细胞可能并不是引起细胞因子风暴的原因。
为了观察单核细胞的表型重塑,作者在分析差异基因时发现相对于健康对照,至少6个COVID-19样品中编码HLA II类分子的8个基因的显着下调(Fig.2d),并且这种下调在所有COVID-19患者中均显着,但在通气依赖患者中可能更明显(Fig.2e)。由于这种下调可能抑制CD4 + T细胞反应的产生,因此可能削弱老年COVID-19患者的适应性免疫反应。非经典HLA I类基因HLA-E和HLA-F也被下调程度较小且样品较少(Fig.2f),而经典HLA I类基因HLA-A,HLA-B和HLA-C并未持续上调或下调。
在至少一个COVID 19样品中,CD14+ 单核细胞上调了35种I型干扰素(IFN)刺激的基因(ISG)(Fig.2d)。相应地,“IFN Signaling”是CD14+ 单核细胞中第二个高度上调的信号通路(Fig.2g)。对CD14+ 单核细胞中上游调节因子的分析表明,相对于其余COVID-19供体,供体C2、C3和C7中没有预测的IFN和IFN调节因子(IRF)高表达(Fig.2h)。我们通过数据集中已知的人类I型IFN刺激基因对数据集中的CD14+ 单核细胞进行了评分,同样得出了相同的结论。通气/ARDS不能解释ISG的差异性特征(Fig.2h,i),但是较高的ISG分数与年龄呈正相关,与发烧时间距离呈负相关(图2j)。在两次采样的患者中(C1),在两次采样之间的间隔48小时内,ISG模块评分显着下降,在此期间,患者失去代偿能力并变得依赖呼吸机(Fig.2i)。这些数据共同表明外周IFN应答、患者年龄和临床严重性之间的相关性。
Figure 2 | Robust HLA class II downregulation and type I IFN-driven inflammatory signatures in monocytes are characteristics of SARS-CoV-2 infection.
a, UMAP embedding of all monocytes colored by sample of origin.
b, UMAP embedding of monocytes colored by CD14 and FCGR3A (CD16, to distinguish between CD14+ and CD16+ monocytes), HLA-DPB1 and HLA-DMA (illustrating HLA class II downregulation in COVID-19 patients), and S100A9 and IFI27 (demonstrating canonical inflammatory signatures in COVID-19 patients).
c, UMAP embedding of monocytes colored by genes encoding pro-inflammatory cytokines previously reported to be produced by circulating monocytes in severe COVID-19, namely TNF, IL6, IL1B, CCL3, CCL4, and CXCL2.
d, g, h, Heatmaps of (d) DE genes, (g) differentially regulated canonical pathways, and (h) differentially regulated predicted upstream regulators between CD14+ monocytes of each donor compared to CD14+ monocytes of all healthy controls. d is colored by average log(fold-change), while g and h are colored by z-score. All displayed genes, pathways, and regulators are statistically significant at the p<0.05 confidence level. The (d) 50 genes, (g) 25 pathways, or (h) 50 regulators with the highest absolute average log(fold-change) or z-score across all donors all labeled. Genes with a net positive average log(fold-change) or z-score are labeled in red; genes with a net negative average log(fold-change) or z-score are labeled in blue.
e, Box plot showing the mean HLA Class II module score of CD14+ monocytes from each sample, colored by healthy donors (blue), non-ventilated COVID-19 patients (orange), or ventilated COVID-19 patients (red). Shown are exact p values by Wilcoxon rank sum test.
f, Dot plot depicting percent expression and average expression of all detected HLA genes in CD14+monocytes by donor.
i, Box plot showing the IFNA module score of each cell, colored by healthy donors (blue), non-ventilated COVID-19 patients (orange), or ventilated COVID-19 patients (red).
j, Scatter plots depicting the correlation between the mean ISG module score of each sample and the patient age (top) and time-distance to first measured or reported fever (bottom).
????滑动查看????
COVID-19中外周血NK细胞表型的异质性
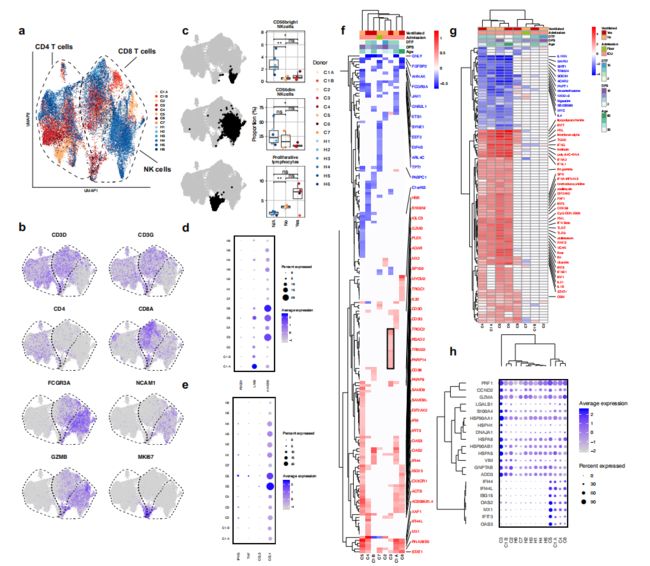
接下来,我们分析了COVID-19患者样品中T和NK淋巴细胞的转录组,因为与健康对照组相比,这些细胞似乎存在明显的表型偏移(Fig.1a,b)。在CD4+ T, CD8+ T和NK cells (Fig. 3a, b)中作者发现,通过细胞介导的细胞毒性有助于抗病毒宿主防御的CD56dim NK细胞在呼吸机依赖的患者中耗竭,而CD56bright NK细胞,被认为是IFN-γ和TNF-α18的强大产生者,在所有COVID 19样品中都显著耗竭(Fig.3c)。
在NK与T细胞中也没有观察到促炎因子的大量表达 (Fig. 3e) ,与健康对照组相比,所有其他COVID-19患者在CD8+ T或NK细胞中均未表达更高水平的CCL3,CCL4,IFNG或TNF(Fig. 3e)。外周T和NK细胞不存在促炎细胞因子表达,这再次表明外周白细胞可能并不是导致COVID-19中的细胞因子风暴的原因。
为了进一步描述COVID-19中T和NK细胞的表型,作者通过每个COVID-19患者样本相对于所有健康对照组的DE基因识别丰富的基因通路和上游调控因子。在COVID-19例患者中,NK细胞表现出明显的异质性反应(Fig.3f)。最常见的下调基因包括FCGR3A、AHNAK、FGFBP2,这些基因与外周血NK细胞的成熟有关。最常见的上调基因包括ISGs和NK细胞活化基因,如PLEK和CD38。作者观察到在CD4+和CD8+ T细胞中DE基因的类似异质性,其中最常见的上调基因是ISGs。
同时对预测的上游调节因子的分析表明,在NK细胞、CD4+ 和CD8+ T细胞中,有一半的COVID-19分析样品中没有明显的干扰素驱动效应(Fig.3g)。尽管最广泛的NK细胞表型转移发生在具有强IFN标记的供体中(Fig.3f,g),不过C3患者中IFN标记极少(Fig.3f框内),作者仍从C3鉴定了一组表达上调的基因,这些基因在其他COVID-19患者中均未上调,包括有NK细胞毒性介质PRF1和GZMB,以及6种热激蛋白(Fig.3h)。从患者C3的CD8+ T细胞中也鉴定到了一组相似的基因,主要由热激蛋白组成。这些结果共同表明,外周血T和NK淋巴细胞的表型在COVID-19中异质重塑。
Figure 3 | Heterogeneous patterns of NK cell exhaustion and interferon response in COVID-19.
a, UMAP embedding of CD4+ T cells, CD8+ T cells, and NK cells colored by sample of origin.
b, UMAP embedding colored by lineage genes (CD3D, CD3G, CD4, CD8A, FCGR3A, and NCAM1) and selected functional/phenotypic markers (GZMB and MKI67).
c, Box plots depicting proportions of CD56dim NK cells, CD56bright NK cells, and proliferating lymphocytes among total T and NK cells by sample of origin. The cells used to calculate each proportion are highlighted in bold black in the adjacent UMAP embeddings. , p<0.01; *, p<0.05; n.s., p>0.05 by Wilcoxon rank sum test.
d, Dot plot showing the percent and average expression of three canonical markers of NK cell exhaustion: LAG3, PDCD1 (encoding PD-1), and HAVCR2 (encoding Tim-3).
e, Dot plot showing the percent and average expression of four canonical NK cell cytokines (CCL3, CCL4, IFNG, and TNF) by NK cells.
f, g, Heatmaps of (f) DE genes and (g) differentially regulated predicted upstream regulators between NK cells of each COVID-19 sample compared to NK cells of all healthy controls. As in Fig. 2, f is colored by average log(fold-change), while g is colored by z-score. All displayed genes and regulators are statistically significant at the p<0.05 confidence level. The 50 genes or regulators with the highest absolute average log(fold-change) or z-score across all donors all labeled. Genes with a net positive average log(fold-change) or z-score are labeled in red; genes with a net negative average log(fold-change) or z-score are labeled in blue. f, Genes that are selectively upregulated by the NK cells of donor C3 are boxed in black.
h, Dot plot depicting the percent and average expression of C3 NK cell-specific genes boxed in (f) as well as selected ISGs upregulated by multiple COVID-19 patients.
????滑动查看????
B细胞中新的粒细胞表型是出现ARDS的重症COVID-19患者的重要特征
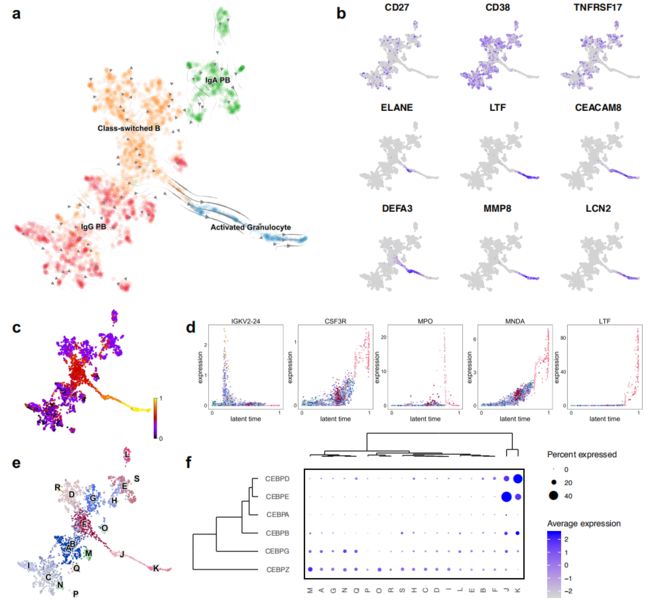
接下来,作者分析了浆母细胞,class switched B cells和活化的粒细胞的表型,UMAP图表明其存在一定的相关性(Fig.1b)。活化的粒细胞似乎是从class-switched B cells线性投射的,表明两种细胞类型之间存在连续的细胞表型。作者通过RNA velocity发现从class-switched B cells到活化粒细胞的线性过渡(Fig.4a), 沿着这个分化轨迹的细胞失去了典型的浆细胞标记基因CD27,CD38和TNFRSF17(编码BCMA)的表达,取而代之的是编码中性粒细胞颗粒蛋白和其他粒细胞标记基因(包括ELANE,LTF和DEFA3)的表达(Fig.4b)。尽管此连续性开始时的细胞是通过Ig基因的表达来定义,但随着时间的增长,粒细胞标志物(如CSF3R和MNDA)(编码髓系核分化抗原)会被上调(Fig.4d)。
作者最后使用NicheNet(a technique that uses gene expression data to discover the putative ligand-target links mediating downstream transcriptional changes),表明如果在该数据集中,EGF和IL24的表达均有限,并且这些细胞因子驱动活化的粒细胞分化,那么它们可能是由外周血中不存在的细胞所产生。
Figure 4 | Activated granulocytes are characteristic of severe COVID-19 patients and differentiate from class-switched B cells
a, UMAP embedding of plasmablasts, class-switched B cells, and activated granulocytes, colored by annotated cell type and overlaid with RNA velocity stream.
b, UMAP embedding colored by canonical plasmablast markers (CD27, CD38, and TNFRSF17) and markers of activated granulocytes (ELANE, LTF, CEACAM8, DEFA3, MMP8, and LCN2).
c, UMAP embedding colored by inferred latent time.
d, Scatter plots showing expression of a selection of cluster-defining genes across inferred latent time.
e, UMAP embedding colored by orthogonally-generated clusters.
f, Dot plot depicting expression of CEBP family members in each
????滑动查看????
校对 | 排版:生信宝典
你可能还想看
利用单细胞公共数据对新冠受体ACE2的研究进展
最新进展!单细胞数据显示ACE2在鼻腔、肾脏、睾丸均有分布!
大联合 - 单细胞测序在新冠肺炎研究中的应用进展 (视频+PPT)
第一篇新冠单细胞文献!|解读
往期精品(点击图片直达文字对应教程)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
后台回复“生信宝典福利第一波”或点击阅读原文获取教程合集
![]()
![]()
![]()