荷兰一项随机对照试验显示, 已获持续6个月临床缓解或低疾病活动度的RA患者在停用TNFi的6个月内约三分之一病情复发
Moghadam HG, et al. ACR 2015. Abstract ID: 1042.
背景与目的:TNF拮抗剂(TNFi)是RA的有效治疗手段。目前尚不清楚已获临床缓解或稳定的低疾病活动度(LDA)的患者是否需要继续使用TNFi, 多药合用以及TNFi的高药费使得探讨这一问题变得非常重要。本研究旨在阐明, 如果RA患者停用TNFi并且因此而出现病情复发, 是否可以安全、有效的重新启动TNFi治疗。
方法:这是一项探讨治疗范式的多中心、开放标签、随机对照临床试验。入组标准如下, 患者符合ACR 1987分类标准, 应用TNFi至少1年, 最近6个月DMARDs剂量稳定且 DAS28<3.2。按2:1的比例将患者随机分入TNFi停药组或TNFi继续应用组。病情复发的定义: DAS28≥3.2且较前次评估相比的增幅≥0.6。如果TNFi停药组患者复发, 经风湿病医生评估后可重新启动TNFi治疗。
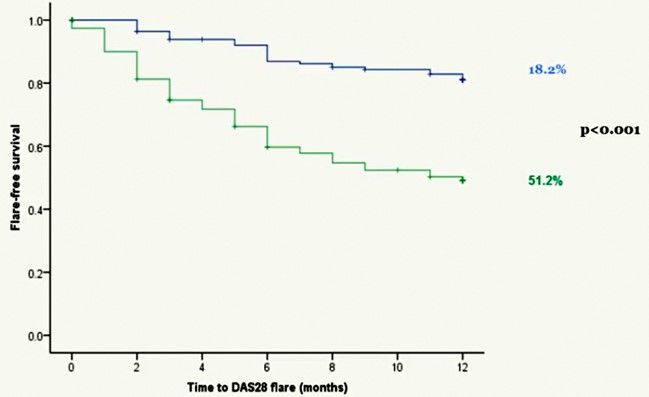
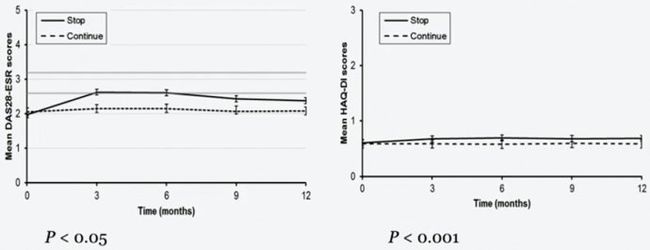
结果:来自47各中心的共817例患者入组, 按2:1的比例随机分入TNFi停药组(531例, 65%)和继续治疗组(286 例, 35%)。6月间病情复发情况为TNFi停药组212/531例(31.5%), 继续治疗组36/286例(9.8%)。12月间, TNFi停药组病情复发有267/531例(50.3%), 继续治疗组有52/286例(18.2%)。TNFi停药组和继续治疗组的复发风险之比为3.41(95%CI: 2.53-4.59)。在整个随访过程中, TNFi停药组DAS28均值升高, 与继续治疗组相比有统计学显著意义(p<0.001)。195例TNFi停药组患者在停药26周内重启TNFi, 其中165例患者(84.6%)在重治6个月内再获LDA, 再获LDA的平均疗程为12周(95% CI: 10.8-13.2).
分析严重不良事件(SAE)发现, TNF继续治疗组有1例死亡(0.3%), 而停药组为零; 因感染而住院的例数分别为TNFi停药组11例(2%)、继续治疗组4例(1.4%)。停药组患者重启TNFi后未发生过敏反应。
结论:对于已获临床缓解或稳定的低疾病活动度的RA患者, 停用TNFi治疗的复发风险显著高于继续接受TNFi治疗的患者。
图1. 分析12月间无复发的Kaplan-Meier生存曲线
[注: 以下图片是ACR2015年会现场演讲拍照。现场演讲所示1年间TNFi停药组和继续治疗组的复发率分别为:272/531(51.2%), 52/286(18.2%, p<0.001), 即现场演讲所示TNFi停药组1年复发率有别于ACR官网之前录入的文摘报道。]
图2. 12月间TNFi停药组与继续治疗组的DAS28均值与HAQ-DI均值的变化
原文如下。
1042 - Pragmatic Multicenter Open-Label RandomizedControlled Trial of Stopping TNF-Inhibitors in Rheumatoid Arthritis Patientsin Remission or Stable Low Disease Activity in the Netherlands
Sunday, November 8, 2015: 5:15 PM
South - Hall A (The Moscone Center)
Presentation Number:1042
Marjan Ghiti Moghadam1,Harald E. Vonkeman1, Peter M. ten Klooster2, Janneke Tekstra3, Dirkjan van Schaardenburg4, Mirian Starmans-kool5, Elisabeth Brouwer6, Reinhard Bos7, Willem F. Lems8, Edgar Colin9, Cornelia F. Allaart10, Inger L. Meek11, Robert B.M. Landewé12, Hein J. Bernelot Moens13, Piet van Riel11, Mart A.F.J. van de Laar1, Tim Jansen14and on behalf of the Dutch National POET Collaboration.,1Medisch Spectrum Twente - Arthritis Center Twente, Enschede, Netherlands,2University of Twente, Enschede, Netherlands,3University Medical Center Utrecht, Utrecht, Netherlands,4Jan van Breemen Research Institute, Amsterdam, Netherlands,5Orbis Medical Center, Geleen-Sittard, Netherlands,6University Medical center Groningen, Groningen, Netherlands,7Medical Center Leeuwarden, Leeuwarden, Netherlands,8Amsterdam Rheumatology and immunology Center, VU University medical center, Amsterdam, Netherlands,9Erasmus MC, Rotterdam, Netherlands,10Department of Rheumatology, Leiden Universitary Medical Center, Leiden, Netherlands,11Radboud University Nijmegen Medical Centre, Nijmegen, Netherlands,12Amsterdam Rheumatology Center, Amsterdam, Netherlands,13Ziekenhuisgroep Twente, Almelo, Netherlands,14VieCuri Medical Center, Venlo, Netherlands
Background/Purpose:TNF-inhibitors (TNFi) are effective treatments of rheumatoid arthritis (RA). It is not clear if patients in remission or stable low disease activity need to continue their TNFi. Issues of polypharmacy and the relatively high costs of TNFi make it important to examine whether patients in remission or stable low disease activity can stop their TNFi. If patients stop TNFi and consequently flare, it is unclear whether TNFi can be restarted effectively and safely.
Methods:Pragmatic multicenter open-label randomized controlled trial. Inclusion criteria: patients diagnosed with RA according to the ACR 1987 criteria, patients using a TNFi for at least 1 year with stable dose DMARDs over the last 6 months, DAS28 <3.2 over the last 6 months. Patients were randomized to either stop or continue their current TNFi in a 2:1 ratio. Flare was defined as DAS28 ≥3.2 with an increase ≥0.6 compared to the previous DAS28. In case of flare in the stop group, TNFi could be restarted at the discretion of the treating rheumatologist.
Results:In total 817 patients from 47 centers were included: 531 patients (65%) in the stop group and 286 patients (35%) in the continuation group. At 6 months, significantly more patients in the stop group (212/531 [31.5%]) had experienced a flare than in the continuation group (36/286 [9.8%], p<0.001). At 12 months these were 267/531 [50.3%] vs 52/286 [18.2%], respectively (p<0.001). The hazard ratio for flare after stopping TNFi was 3.41 (95% CI: 2.53–4.59). Mean DAS28 scores in the stop group were significantly increased throughout the follow-up period compared with the continuation group (p<0.001). Of the 195 patients that restarted TNFi within 26 weeks, 165 (84.6%) had regained low disease activity (DAS28 < 3.2) 6 months later and median time to regained low disease activity was 12 weeks (95% Cl: 10.8-13.2).
SAEs in stop vs. continuation groups: Deaths0vs. 1 (0.3%) and hospitalization due to infections 11 (2%) vs. 4 (1,4%), respectively. There were no allergic reactions among the patients in the stop group that restarted TNFi.
Conclusion:Stopping TNFi treatment in RA patients in remission or stable low disease activity results in substantially more flares than continuing.