【lighting】Color and Vision
The Electromagnetic and Visible Spectra
原文:http://www.physicsclassroom.com/class/light/Lesson-2/The-Electromagnetic-and-Visible-Spectra
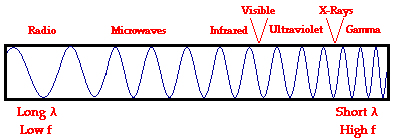
As discussed in Unit 10 of The Physics Classroom Tutorial, electromagnetic waves are waves that are capable of traveling through a vacuum. Unlikemechanical waves that require a medium in order to transport their energy, electromagnetic waves are capable of transporting energy through the vacuum of outer space. Electromagnetic waves are produced by a vibrating electric charge and as such, they consist of both an electric and a magnetic component. The precise nature of such electromagnetic waves is not discussed in The Physics Classroom Tutorial. Nonetheless, there are a variety of statements that can be made about such waves.
Electromagnetic waves exist with an enormous range of frequencies. This continuous range of frequencies is known as theelectromagnetic spectrum. The entire range of the spectrum is often broken into specific regions. The subdividing of the entire spectrum into smaller spectra is done mostly on the basis of how each region of electromagnetic waves interacts with matter. The diagram below depicts the electromagnetic spectrum and its various regions. The longer wavelength, lower frequency regions are located on the far left of the spectrum and the shorter wavelength, higher frequency regions are on the far right. Two very narrow regions within the spectrum are the visible light region and the X-ray region. You are undoubtedly familiar with some of the other regions of the electromagnetic spectrum.
Visible Light Spectrum
The focus of Lesson 2 will be upon the visible light region - the very narrow band of wavelengths located to the right of the infrared region and to the left of the ultraviolet region. Though electromagnetic waves exist in a vast range of wavelengths, our eyes are sensitive to only a very narrow band. Since this narrow band of wavelengths is the means by which humans see, we refer to it as thevisible light spectrum. Normally when we use the term "light," we are referring to a type of electromagnetic wave that stimulates the retina of our eyes. In this sense, we are referring to visible light, a small spectrum from the enormous range of frequencies of electromagnetic radiation. This visible light region consists of a spectrum of wavelengths that range from approximately 700 nanometers (abbreviated nm) to approximately 400 nm. Expressed in more familiar units, the range of wavelengths extends from 7 x 10-7 meter to 4 x 10-7 meter. This narrow band of visible light is affectionately known asROYGBIV.
Each individual wavelength within the spectrum of visible light wavelengths is representative of a particular color. That is, when light of that particular wavelength strikes the retina of our eye, we perceive that specific color sensation. Isaac Newton showed thatlight shining through a prism will be separated into its different wavelengths and will thus show the various colors that visible light is comprised of. The separation of visible light into its different colors is known asdispersion. Each color is characteristic of a distinct wavelength; and different wavelengths of light waves will bend varying amounts upon passage through a prism. For these reasons, visible light is dispersed upon passage through a prism. Dispersion of visible light produces the colors red (R), orange (O), yellow (Y), green (G), blue (B), and violet (V). It is because of this that visible light is sometimes referred to as ROY G. BIV.(Incidentally, the indigo is not actually observed in the spectrum but is traditionally added to the list so that there is a vowel in Roy's last name.) The red wavelengths of light are the longer wavelengths and the violet wavelengths of light are the shorter wavelengths. Between red and violet, there is a continuous range or spectrum of wavelengths. The visible light spectrum is shown in the diagram below.
that specific color sensation. Isaac Newton showed thatlight shining through a prism will be separated into its different wavelengths and will thus show the various colors that visible light is comprised of. The separation of visible light into its different colors is known asdispersion. Each color is characteristic of a distinct wavelength; and different wavelengths of light waves will bend varying amounts upon passage through a prism. For these reasons, visible light is dispersed upon passage through a prism. Dispersion of visible light produces the colors red (R), orange (O), yellow (Y), green (G), blue (B), and violet (V). It is because of this that visible light is sometimes referred to as ROY G. BIV.(Incidentally, the indigo is not actually observed in the spectrum but is traditionally added to the list so that there is a vowel in Roy's last name.) The red wavelengths of light are the longer wavelengths and the violet wavelengths of light are the shorter wavelengths. Between red and violet, there is a continuous range or spectrum of wavelengths. The visible light spectrum is shown in the diagram below.

When all the wavelengths of the visible light spectrum strike your eye at the same time, white is perceived. The sensation of white is not the result of a single color of light. Rather, the sensation of white is the result of a mixture of two or more colors of light. Thus, visible light - the mix of ROYGBIV - is sometimes referred to aswhite light. Technically speaking, white is not a color at all - at least not in the sense that there is a light wave with a wavelength that is characteristic of white. Rather, white is the combination of all the colors of the visible light spectrum. If all the wavelengths of the visible light spectrum give the appearance of white, then none of the wavelengths would lead to the appearance of black. Once more, black is not actually a color. Technically speaking, black is merely the absence of the wavelengths of the visible light spectrum. So when you are in a room with no lights and everything around you appears black, it means that there are no wavelengths of visible light striking your eye as you sight at the surroundings.
What Wavelength Goes With a Color?
原文:http://science-edu.larc.nasa.gov/EDDOCS/Wavelengths_for_Colors.html#green
| Our eyes are sensitive to light which lies in a very small region of theelectromagnetic spectrum labeled "visible light". This "visiblelight" corresponds to a wavelength range of 400 - 700 nanometers (nm) anda color range of violet through red. The human eye is not capable of"seeing" radiation with wavelengths outside the visible spectrum. Thevisible colors from shortest to longest wavelength are: violet, blue, green,yellow, orange, and red. Ultraviolet radiation has a shorter wavelength thanthe visible violet light. Infrared radiation has a longer wavelength thanvisible red light. The white light is a mixture of the colors of the visiblespectrum. Black is a total absence of light. Earth's most important energy source is the Sun. Sunlight consists of theentire electromagnetic spectrum. Learn more:
|
(Wavelength image from Universe by Freedman and Kaufmann.) |
Violet LightThe visible violet light has a wavelength of about 400 nm. Within thevisible wavelength spectrum, violet and blue wavelengths are scattered moreefficiently than other wavelengths. The sky looks blue, not violet, because oureyes are more sensitive to blue light (the sun also emits more energy as bluelight than as violet). |
|
Indigo LightThe visible indigo light has a wavelength of about 445 nm. |
|
Blue LightThe visible blue light has a wavelength of about 475 nm. Because the bluewavelengths are shorter in the visible spectrum, they are scattered moreefficiently by the molecules in the atmosphere. This causes the sky to appearblue. |
|
Green LightThe visible green light has a wavelength of about 510 nm. Grass, for example,appears green because all of the colors in the visible part of the spectrumare absorbed into the leaves of the grass except green. Green is reflected,therefore grass appears green. |
|
Yellow LightThe visible yellow light has a wavelength of about 570 nm. Low-pressuresodium lamps, like those used in some parking lots, emit a yellow (wavelength589 nm) light. |
|
Orange LightThe visible orange light has a wavelength of about 590 nm. |
|
Red LightThe visible red light has a wavelength of about 650 nm. At sunrise andsunset, red or orange colors are present because the wavelengths associatedwith these colors are less efficiently scattered by the atmosphere than theshorter wavelength colors (e.g., blue and purple). A large amount of blue andviolet light has been removed as a result of scattering and the longwavecolors, such as red and orange, are more readily seen. |
Colors We Can't See
There are many wavelengths in the electromagnetic spectrum the human eyecannot detect.
Energy with wavelengths too short for humans to see
Energy with wavelengths too short to see is "bluer than blue". Light with such short wavelengths is called "Ultraviolet" light.
How do we know this light exists? One way is that this kind of light causessunburns. Our skin is sensitive to this kind of light. If we stay out in thislight without sunblock protection, our skin absorbs this energy. After theenergy is absorbed, it can make our skin change color ("tan") or itcan break down the cells and cause other damage.
Energy with wavelengths too long for humans to see
Energy whose wavelength is too long to see is "redder than red". Light with such long wavelengths is called "Infrared" light. The term "Infra-" means "lower than".
How do we know this kind of light exists? One way is that we can feel energywith these wavelengths such as when we sit in front of a campfire or when weget close to a stove burner. Scientists likeSamuel Pierpont Langley passed light througha prism and discovered that the infrared light the scientists could not seebeyond red could make other things hot.
Very long wavelengths of infrared light radiate heat to outer space. This radiation is important to the Earth's energy budget. If this energy did not escape to space, the solar energy that the Earth absorbs would continue toheat the Earth.
Visible Light and the Eye's Response
- The Electromagnetic and Visible Spectra
- Visible Light and the Eye's Response
- Light Absorption, Reflection, and Transmission
- Color Addition
- Color Subtraction
- Blue Skies and Red Sunsets
As mentioned in the first section of Lesson 2, our eyes are sensitive to a very narrow band of frequencies within the enormous range of frequencies of the electromagnetic spectrum. This narrow band of frequencies is referred to as the visible light spectrum. Visible light - that which is detectable by the human eye - consists of wavelengths ranging from approximately 780 nanometer (7.80 x 10-7 m) down to 390 nanometer (3.90 x 10-7 m). Specific wavelengths within the spectrum correspond to a specific color based upon how humans typically perceive light of that wavelength. The long wavelength end of the spectrum corresponds to light that is perceived by humans to be red and the short wavelength end of the spectrum corresponds to light that is perceived to be violet. Other colors within the spectrum include orange, yellow, green and blue. The graphic below depicts the approximate range of wavelengths that are associated with the various perceived colors within the spectrum.
Color Cones
Color can be thought of as a psychological and physiological response to light waves of a specific frequency or set of frequencies impinging upon the eye. An understanding of the human response to color demands that one understand the biology of the eye. Light that enters the eye through the pupil ultimately strikes the inside surface of the eye known as the retina. The retina is lined with a variety of light sensing cells known as rods and cones. While the rods on the retina are sensitive to the intensity of light, they cannot distinguish between lights of different wavelengths. On the other hand, the cones are the color-sensing cells of the retina. When light of a given wavelength enters the eye and strikes the cones of the retina, a chemical reaction is activated that results in an electrical impulse being sent along nerves to the brain. It is believed that there are three kinds of cones, each sensitive to its own range of wavelengths within the visible light spectrum. These three kinds of cones are referred to as red cones, green cones, and blue cones because of their respective sensitivity to the wavelengths of light that are associated with red, green and blue. Since the red cone is sensitive to a range of wavelengths, it is not only activated by wavelengths of red light, but also (to a lesser extent) by wavelengths of orange light, yellow light and even green light. In the same manner, the green cone is most sensitive to wavelengths of light associated with the color green. Yet the green cone can also be activated by wavelengths of light associated with the colors yellow and blue. The graphic below is a sensitivity curve that depicts the range of wavelengths and the sensitivity level for the three kinds of cones.
The cone sensitivity curve shown above helps us to better understand our response to the light that is incident upon the retina. While the response is activated by the physics of light waves, the response itself is both physiological and psychological. Suppose that white light - i.e., light consisting of the full range of wavelengths within the visible light spectrum - is incident upon the retina. Upon striking the retina, the  physiological occurs: photochemical reactions occur within the cones to produce electrical impulses that are sent along nerves to the brain. The cones respond to the incident light by sending a message forward to brain, saying, "Light is hitting me." Upon reaching the brain, the psychological occurs: the brain detects the electrical messages being sent by the cones and interprets the meaning of the messages. The brain responds by saying "it is white." For the case of white light entering the eye and striking the retina, each of the three kinds of cones would be activated into sending the electrical messages along to the brain. And the brain recognizes that the messages are being sent by all three cones and somehow interprets this to mean that white light has entered the eye.
physiological occurs: photochemical reactions occur within the cones to produce electrical impulses that are sent along nerves to the brain. The cones respond to the incident light by sending a message forward to brain, saying, "Light is hitting me." Upon reaching the brain, the psychological occurs: the brain detects the electrical messages being sent by the cones and interprets the meaning of the messages. The brain responds by saying "it is white." For the case of white light entering the eye and striking the retina, each of the three kinds of cones would be activated into sending the electrical messages along to the brain. And the brain recognizes that the messages are being sent by all three cones and somehow interprets this to mean that white light has entered the eye.
Now suppose that light in the yellow range of wavelengths (approximately 577 nm to 597 nm) enters the eye and strikes the retina. Light with these wavelengths would activate both the green and the red cones of the retina. Upon striking the retina, the physiological occurs: electrical messages are sent by both the red and the green cones to the brain. Once received by the brain, the psychological occurs: the brain recognizes that the light has activated both the red and the green cones and somehow interprets this to mean that the object is yellow. In this sense, the yellow appearance of objects is simply the result of yellow light from the object entering our eye and stimulating the red and the green cones simultaneously.
If the appearance of yellow is perceived of an object when it activates the red and the green cones simultaneously, then what appearance would result if two overlapping red and green spotlights entered our eye? Using the same three-cone theory, we could make some predictions of the result. Red light entering our eye would mostly activate the red color cone; and green light entering our eye would mostly activate the green color cone. Each cone would send their usual electrical messages to the brain. If the brain has been psychologically trained to interpret these two signals to mean "yellow", then the brain would perceive the overlapping red and green spotlights to appear as yellow. To the eye-brain system, there is no difference in the physiological and psychological response to yellow light and a mixing of red and green light. The brain has no means of distinguishing between the two physical situations.
In a technical sense, it is really not appropriate to refer to light as being colored. Light is simply a wave with a specific wavelength or a mixture of wavelengths; it has no color in and of itself. An object that is emitting or reflecting light to our eye appears to have a specific color as the result of the eye-brain response to the  wavelength. So technically, there is really no such thing as yellow light. Rather, there is light with a wavelength of about 590 nm that appears yellow. And there is also light with a mixture of wavelengths of about 700 nm and 530 nm that together appears yellow. The yellow appearance of these two clearly different light sources can be traced to the physiological and psychological response of the eye-brain system, and not to the light itself. So to be technically appropriate, a person would refer to "yellow light" as "light that creates a yellow appearance." Yet, to maintain a larger collection of friendships, a person would refer to "yellow light" as "yellow light."
wavelength. So technically, there is really no such thing as yellow light. Rather, there is light with a wavelength of about 590 nm that appears yellow. And there is also light with a mixture of wavelengths of about 700 nm and 530 nm that together appears yellow. The yellow appearance of these two clearly different light sources can be traced to the physiological and psychological response of the eye-brain system, and not to the light itself. So to be technically appropriate, a person would refer to "yellow light" as "light that creates a yellow appearance." Yet, to maintain a larger collection of friendships, a person would refer to "yellow light" as "yellow light."
In the next several sections of Lesson 2, we will explore these concepts further by introducing three primary colors of light and generating some simple rules for predicting the color appearance of objects in terms of the three primary colors.
Color Addition
- The Electromagnetic and Visible Spectra
- Visible Light and the Eye's Response
- Light Absorption, Reflection, and Transmission
- Color Addition
- Color Subtraction
- Blue Skies and Red Sunsets
Color perception, like sound perception, is a complex subject involving the disciplines of psychology, physiology, biology, chemistry and physics. When you look at an object and perceive a distinct color, you are not necessarily seeing a single frequency of light. Consider for instance that you are looking at a shirt and it appears purple to your eye. In such an instance, there may be several frequencies of light striking your eye with varying degrees of intensity. Yet your eye-brain system interprets the frequencies that strike your eye and the shirt is decoded by your brain as being purple.
Primary Colors of Light
The subject of color perception can be simplified if we think in terms of primary colors of light. We have already learned that white is not a color at all, but rather the presence of all the frequencies of visible light. When we speak of white light, we are referring to ROYGBIV - the presence of the entire spectrum of visible light. But combining the range of frequencies in the visible light spectrum is not the only means of producing white light. White light can also be produced by combining only three distinct frequencies of light, provided that they are widely separated on the visible light spectrum. Any three colors (or frequencies) of light that produce white light when combined with the correct intensity are called primary colors of light. There are a variety of sets of primary colors. The most common set of primary colors is red (R), green (G) and blue (B). When red, green and blue light are mixed or added together with the proper intensity, white (W) light is obtained. This is often represented by the equation below:
In fact, the mixing together (or addition) of two or three of these three primary colors of light with varying degrees of intensity can produce a wide range of other colors. For this reason, many television sets and computer monitors produce the range of colors on the monitor by the use of red, green and blue light-emitting phosphors.
The addition of the primary colors of light can be demonstrated using a light box. The light box illuminates a screen with the three primary colors - red (R), green (G) and blue (B). The lights are often the shape of circles. The result of adding two primary colors of light is easily seen by viewing the overlap of the two or more circles of primary light. The different combinations of colors produced by red, green and blue are shown in the graphic below. (CAUTION: Because of the way that different monitors and different web browsers render the colors on the computer monitor, there may be slight variations from the intended colors.)

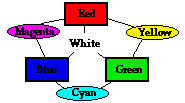
Color Addition Rules
These demonstrations with the color box illustrate that red light and green light add together to produce yellow (Y) light. Red light and blue light add together to produce magenta (M) light. Green light and blue light add together to produce cyan (C) light. And finally, red light and green light and blue light add together to produce white light. This is sometimes demonstrated by the following color equations and graphic:
|
R + G = Y |
|
Yellow (Y), magenta (M) and cyan (C) are sometimes referred to as secondary colors of light since they can be produced by the addition of equal intensities of two primary colors of light. The addition of these three primary colors of light with varying degrees of intensity will result in the countless other colors that we are familiar (or unfamiliar) with.
Color Subtraction
- The Electromagnetic and Visible Spectra
- Visible Light and the Eye's Response
- Light Absorption, Reflection, and Transmission
- Color Addition
- Color Subtraction
- Blue Skies and Red Sunsets
The previous lesson focused on the principles of color addition. These principles govern the perceived color resulting from the mixing of different colors of light. Principles of color addition have important applications to color television, color computer monitors and on-stage lighting at the theaters. Each of these applications involves the mixing or addition of colors of light to produce a desired appearance. Our understanding of color perception would not be complete without an understanding of the principles of color subtraction. In this part of Lesson 2, we will learn how materials that have been permeated by specific pigments will selectively absorb specific frequencies of light in order to produce a desired appearance.
We have already learned that materials contain atoms that are capable of selectively absorbing one or more frequencies of light. Consider a shirt made of a material that is capable of absorbing blue light. Such a material will absorb blue light (if blue light shines upon it) and reflect the other frequencies of the visible spectrum. What appearance will such a shirt have if illuminated with white light and how can we account for its appearance? To answer this question (and any other similar question), we will rely on our understanding of the three primary colors of light (red, green and blue) and the three secondary colors of light (magenta, yellow and cyan).
 The Process of Color Subtraction
The Process of Color Subtraction
To begin, consider white light to consist of the three primary colors of light - red, green and blue. If white light is shining on a shirt, then red, green and blue light is shining on the shirt. If the shirt absorbs blue light, then only red and green light will be reflected from the shirt. So while red, green and blue light shine upon the shirt, only red and green light will reflect from it. Red and green light striking your eye always gives the appearance of yellow; for this reason, the shirt will appear yellow. This discussion illustrates the process of color subtraction. In this process, the ultimate color appearance of an object is determined by beginning with a single color or mixture of colors and identifying which color or colors of light are subtracted from the original set. The process is depicted visually by diagram at the right. Furthermore, the process is depicted in terms of an equation in the space below.
![]() Now suppose that cyan light is shining on the same shirt - a shirt made of a material that is capable of absorbing blue light. What appearance will such a shirt have if illuminated with cyan light and how can we account for its appearance? To answer this question, the process of color subtraction will be applied once more. In this situation, we begin with only blue and green primary colors of light (recall that cyan light consists of blue and green light). From this mixture, we must subtract blue light. After the subtractive process, only green light remains. Thus, the shirt will appear green in the presence of cyan light. Observe the representation of this by the diagram at the right and the equation below.
Now suppose that cyan light is shining on the same shirt - a shirt made of a material that is capable of absorbing blue light. What appearance will such a shirt have if illuminated with cyan light and how can we account for its appearance? To answer this question, the process of color subtraction will be applied once more. In this situation, we begin with only blue and green primary colors of light (recall that cyan light consists of blue and green light). From this mixture, we must subtract blue light. After the subtractive process, only green light remains. Thus, the shirt will appear green in the presence of cyan light. Observe the representation of this by the diagram at the right and the equation below.
C - B = (G + B) - B = G
From these two examples, we can conclude that a shirt that looks yellow when white light shines upon it will look green when cyan light shines upon it. This confuses many  students of physics, especially those who still believe that the color of a shirt is in the shirt itself. This is the misconception that was targeted earlier in Lesson 2 as we discussed how visible light interacts with matter to produce color. In that part of Lesson 2, it was emphasized that the color of an object does not reside in the object itself. Rather, the color is in the light that shines upon the object and that ultimately becomes reflected or transmitted to our eyes. Extending this conception of color to the above two scenarios, we would reason that the shirt appears yellow if there is some red and green light shining upon it. Yellow light is a combination of red and green light. A shirt appears yellow if it reflects red and green light to our eyes. In order to reflect red and green light, these two primary colors of light must be present in the incident light.
students of physics, especially those who still believe that the color of a shirt is in the shirt itself. This is the misconception that was targeted earlier in Lesson 2 as we discussed how visible light interacts with matter to produce color. In that part of Lesson 2, it was emphasized that the color of an object does not reside in the object itself. Rather, the color is in the light that shines upon the object and that ultimately becomes reflected or transmitted to our eyes. Extending this conception of color to the above two scenarios, we would reason that the shirt appears yellow if there is some red and green light shining upon it. Yellow light is a combination of red and green light. A shirt appears yellow if it reflects red and green light to our eyes. In order to reflect red and green light, these two primary colors of light must be present in the incident light.
![]() Test your understanding of these principles of color subtraction by determining the color appearance of the same shirts if illuminated with other colors of light. Be sure to begin by determining the primary color(s) of light that are incident upon the object and then subtracting the absorbed color from the incident color(s).
Test your understanding of these principles of color subtraction by determining the color appearance of the same shirts if illuminated with other colors of light. Be sure to begin by determining the primary color(s) of light that are incident upon the object and then subtracting the absorbed color from the incident color(s).
Complementary Colors and Color Subtraction
In the above examples, the paper absorbed blue light. Paper that absorbs blue light is permeated by a pigment known as a yellow pigment. While most pigments absorb more than a single frequency (and are known as compound pigments), it becomes convenient for our discussion to keep it simple by assuming that a yellow pigment absorbs a single frequency. A pigment that absorbs a single frequency is known as a pure pigment. The following rule will assist in understanding what colors of light are absorbed by which pigments.
Pigments absorb light. Pure pigments absorb a single frequency or color of light. The color of light absorbed by a pigment is merely the complementary color of that pigment.
Thus, pure blue pigments absorb yellow light (which can be thought of as a combination of red and green light). Pure yellow pigments absorb blue light. Pure green pigments absorb magenta light (which can be thought of as a combination of red and blue light). Pure magenta pigments absorb green light. Pure red pigments absorb cyan light (which can be thought of as a combination of blue and green light). And finally, pure cyan pigments absorb red light.
Now lets combine the process of color subtraction with an understanding of complementary colors to determine the color appearance of various sheets of paper when illuminated by various lights. We will investigate three examples.
Example 1 |
Magenta light can be thought of as consisting of red light and blue light. A yellow pigment is capable of absorbing blue light. Thus, blue is subtracted from the light that shines on the paper. This leaves red light. If the paper reflects the red light, then the paper will look red.
Example 2 |
Yellow light can be thought of as consisting of red light and green light. A red pigment is capable of absorbing cyan light. That is, red paper can absorb both green and blue primary colors of light (recall that cyan light is a mixture of green and blue light). So red and green light shine on the paper; and green light is subtracted. (There is no need to subtract blue light since blue light is not shining on the paper.) This leaves red light to be reflected. If the paper reflects the red light, then the paper will look red.
Example 3 |
Yellow light can be thought of as consisting of red light and green light. A blue pigment is capable of absorbing yellow light. That is, blue paper can absorb both red and green primary colors of light (recall that yellow light is a mixture of red and green light). So red and green light shine on the paper; and both the red and the green light are subtracted. There is no color left to be reflected to the eye. Subsequently, the paper appears black.
Flickr Physics Photo
Filters and Color Subtraction
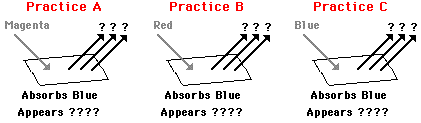
The above discussion applies to the appearance of opaque materials. The distinction between opaque and transparent materials was made earlier in this lesson. Opaque materials selectively absorb one or more frequencies of light and reflect what is not absorbed. In contrast to opaque materials, transparent materials selectively absorb one or more frequencies of light and transmit what is not absorbed. Like opaque materials, transparent materials are permeated by pigments that contain atoms that are capable of absorbing light with a single frequency or even a range of frequencies. Knowing the color(s) of the incident light and the color of light absorbed by the pigment or filter, the process of color subtraction can be applied to determine the color appearance of a transparent material. We will consider three examples in the space below; the examples are visually depicted in the diagrams below.
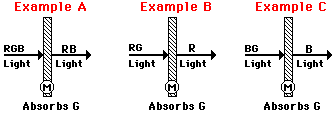
In Example A, white light (i.e., a mixture of red, green and blue) shines upon a magenta filter. Magenta absorbs its complementary color - green. Thus, green is subtracted from white light. That leaves red and blue light to be transmitted by the filter. For this reason, the filter will appear magenta (recall that magenta light is a mixture of red and blue light) when illuminated with white light. This process of color subtraction can be represented by the following equation.
In Example B, yellow light (i.e., a mixture of red and green) shines upon the same magenta filter. Magenta absorbs its complementary color - green. Thus, green is subtracted from yellow light. That leaves red light to be transmitted by the filter. For this reason, the filter will appear red when illuminated with yellow light. This process of color subtraction can be represented by the following equation.
In Example C, cyan light (i.e., a mixture of blue and green) shines upon the same magenta filter. Magenta absorbs its complementary color - green. Thus, green is subtracted from cyan light. That leaves blue light to be transmitted by the filter. For this reason, the filter will appear blue when illuminated with cyan light. This process of color subtraction can be represented by the following equation.
The reasoning modeled in the above three examples can be used in any situation, regardless of the color of the incident light and the color of the filter. As you approach such problems, whether they involve transparent or opaque materials, be sure to think in terms of primary colors of light and to use the logical reasoning steps. Avoid memorizing and avoid shortcuts. If a filter is capable of absorbing a color of light that is not present in the mixture of incident light, then merely disregard that color. Since that color of light is not incident upon the object, it cannot contribute to the color appearance of the object.
We Would Like to Suggest ...
Visit: Colored Filters Interactive
Primary Colors of Paint
A trip to the local newspaper or film developing company will reveal these same principles of color subtraction at work. The three primary colors of paint used by an artist, color printer or film developer are cyan (C), magenta (M), and yellow (Y). Artists, printers, and film developers do not deal directly with light; rather, they must apply paints or dyes to a white sheet of paper. These paints and dyes must be capable of absorbing the appropriate components of white light in order to produce the desired affect. Most artists start with a white canvas and apply paints. These paints have to subtract colors so that you might see the desired image. An artist can create any color by using varying amounts of these three primary colors of paint.
Each primary color of paint absorbs one primary color of light. The color absorbed by a primary color of paint is the complementary color of that paint. The three colors that are primary to an artist (magenta, cyan, and yellow) subtract red, green, and blue individually from an otherwise white sheet of paper. Thus,
Magenta paints absorb green light.
Cyan paints absorb red light.
Yellow paints absorb blue light.
Let's suppose that an artist wishes to use the three primary colors of paint in order to produce a picture of the colorful bird shown at the right. The bird will be painted onto white paper and viewed under white light. It is hoped that the bird will have green tail feathers, a blue lower body, a cyan upper body, a red head, a magenta eye patch, a yellow eye and middle feathers, and a black beak. How can the three primary colors of paint be used to produce such a likeness? And how can we explain the answers in terms of color subtraction?
To produce a green tail, paints must be applied to the tail region in order to absorb red and blue light and leave green to be reflected. Thus, the green tail must be painted using yellow paint (to absorb the blue) and cyan paint (to absorb the red).
To produce a blue lower body, paints must be applied to the lower body region in order to absorb red and green light, leaving blue light to be reflected. Thus, the blue lower body must be painted using magenta paint (to absorb the green) and cyan paint (to absorb the red).
To produce a red head, paints must be applied to the head region in order to absorb blue and green light, leaving red light to be reflected. Thus, the red head must be painted using magenta paint (to absorb the green) and yellow paint (to absorb the blue).
To produce a cyan upper body, paints must be applied to the upper body region in order to absorb red, leaving green and blue light to be reflected. If green and blue light are reflected from the upper body region, it will appear cyan (recall that blue and green light combine to form cyan light). Thus, the cyan upper body must be painted using merely cyan paint (to absorb the red).
To produce a magenta eye patch, paints must be applied to the eye patch region in order to absorb green, leaving red and blue light to be reflected. If red and blue light is reflected from the eye patch region, it will appear magenta (recall that blue and red light combine to form magenta light). Thus, the magenta eye patch must be painted using merely magenta paint (to absorb the green).
To produce a yellow eye and middle feathers, paints must be applied to the eye and middle feather regions in order to absorb blue, leaving red and green light to be reflected. If red and green light is reflected from the eye and middle feather regions, it will appear yellow (recall that red and green light combine to form yellow light). Thus, the yellow eye and middle feathers must be painted using merely yellow paint (to absorb the blue).
This information is summarized in the graphic below.
The process of color subtraction is a useful means of predicting the ultimate color appearance of an object if the color of the incident light and the pigments are known. By using the complementary color scheme, the colors of light that will be absorbed by a given material can be determined. These colors are subtracted from the incident light colors (if present) and the colors of reflected light (or transmitted light) can be determined. Then the color appearance of the object can be predicted.
Blue Skies and Red Sunsets
- The Electromagnetic and Visible Spectra
- Visible Light and the Eye's Response
- Light Absorption, Reflection, and Transmission
- Color Addition
- Color Subtraction
- Blue Skies and Red Sunsets
The sun emits light waves with a range of frequencies. Some of these frequencies fall within the visible light spectrum and thus are detectable by the human eye. Since sunlight consists of light with the range of visible light frequencies, it appears white. This white light is incident towards Earth and illuminates both our outdoor world and the atmosphere that surrounds our planet. As discussed earlier in Lesson 2, the interaction of visible light with matter will often result in the absorption of specific frequencies of light. The frequencies of visible light that are not absorbed are either transmitted (by transparent materials) or reflected (by opaque materials). As we sight at various objects in our surroundings, the color that we perceive is dependent upon the color(s) of light that are reflected or transmitted by those objects to our eyes. So if we consider a green leaf on a tree, the atoms of the chlorophyll molecules in the leaf are absorbing most of the frequencies of visible light (except for green) and reflecting the green light to our eyes. The leaf thus appears green. And as we view the black asphalt street, the atoms of the asphalt are absorbing all the frequencies of visible light and no light is reflected to our eyes. The asphalt street thus appears black (the absence of color). In this manner, the interaction of sunlight with matter contributes to the color appearance of our surrounding world. In this part of Lesson 2, we will focus on the interaction of sunlight with atmospheric particles to produce blue skies and red sunsets. We will attempt to answer these two questions:
- Why are the skies blue?
- Why are the sunsets red?
![]()
Why are the skies blue?
The interaction of sunlight with matter can result in one of three wave behaviors: absorption, transmission, and reflection. The atmosphere is a gaseous sea that contains a variety of types of particles; the two most common types of matter present in the atmosphere are gaseous nitrogen and oxygen. These particles are most effective in scattering the higher frequency and shorter wavelength portions of the visible light spectrum. This scattering process involves the absorption of a light wave by an atom followed by reemission of a light wave in a variety of directions. The amount of multidirectional scattering that occurs is dependent upon the frequency of the light. (In fact, it varies according to f4.) Atmospheric nitrogen and oxygen scatter violet light most easily, followed by blue light, green light, etc. So as white light (ROYGBIV) from the sun passes through our atmosphere, the high frequencies (BIV) become scattered by atmospheric particles while the lower frequencies (ROY) are most likely to pass through the atmosphere without a significant alteration in their direction. This scattering of the higher frequencies of light illuminates the skies with light on the BIV end of the visible spectrum. Compared to blue light, violet light is most easily scattered by atmospheric particles. However, our eyes are more sensitive to light with blue frequencies. Thus, we view the skies as being blue in color.
Why are sunsets red?
Meanwhile, the light that is not scattered is able to pass through our atmosphere and reach our eyes in a rather non-interrupted path. The lower frequencies of sunlight (ROY) tend to reach our eyes as we sight directly at the sun during midday. While sunlight consists of the entire range of frequencies of visible light, not all frequencies are equally intense. In fact, sunlight tends to be most rich with yellow light frequencies. For these reasons, the sun appears yellow during midday due to the direct passage of dominant amounts of yellow frequencies through our atmosphere and to our eyes.

The appearance of the sun changes with the time of day. While it may be yellow during midday, it is often found to gradually turn color as it approaches sunset. This can be explained by light scattering. As the sun approaches the horizon line, sunlight must traverse a greater distance through our atmosphere; this is demonstrated in the diagram below.

As the path that sunlight takes through our atmosphere increases in length, ROYGBIV encounters more and more atmospheric particles. This results in the scattering of greater and greater amounts of yellow light. During sunset hours, the light passing through our atmosphere to our eyes tends to be most concentrated with red and orange frequencies of light. For this reason, the sunsets have a reddish-orange hue. The effect of a red sunset becomes more pronounced if the atmosphere contains more and more particles. The presence of sulfur aerosols (emitted as an industrial pollutant and by volcanic activity) in our atmosphere contributes to some magnificent sunsets (and some very serious environmental problems).
The Wonders of Physics
Photograph of Maui sunset by Becky Henderson