波粒二象性仿真理论(一) Wave Particle Duality Principle
Wave Particle Duality Principle
October 22, 2020 by Electrical4U
With the development of Photoelectric effect, Crompton’s effect and Bohr’s model of atom, the idea of light or in fact radiations in general, being composed of particles or discrete Quanta was gaining wide popularity.
However, the very established Huygen’s Principle and the results of Young’s double slit experiments made it very clear that light was wave and not a flow particles.
随着光电效应、克朗普顿效应和玻尔原子模型的发展,由粒子或离散量子组成的光或实际上是辐射的概念越来越流行。然而,非常成熟的惠根原理和杨氏双缝实验的结果非常清楚地表明,光是波,而不是流动粒子。
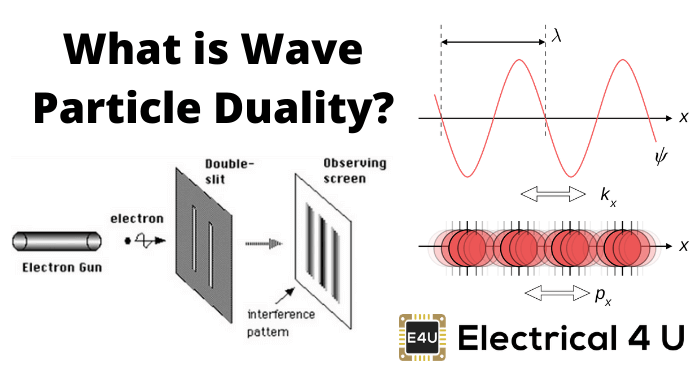
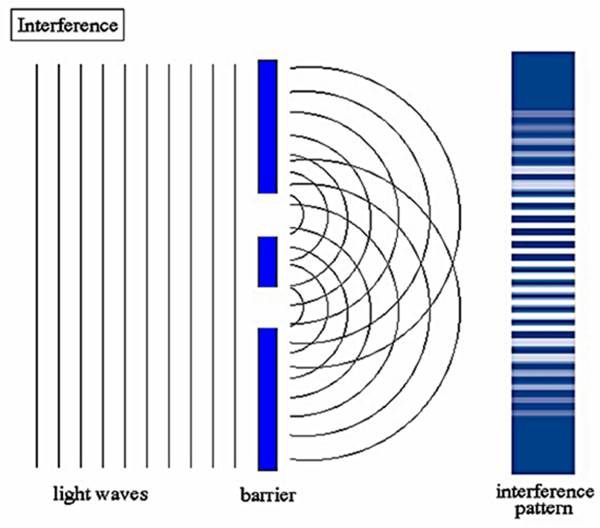
The striking interference pattern observed by passing light through double slits was definitely a result of the wave nature of light. This again gave rise to the controversy of nature of light. In 1704 Newton had also suggested the particle nature of light by his corpuscular theory.
Neither of the two theories were adequate enough to explain all the phenomena associated with light. Thus scientists began to conclude that light has both wave and particle nature. In 1924, a French physicist, Louis de Broglie came up with a theory. He suggested that all particles in this universe is associated with wave nature also, i.e. everything in this world be it a small photon or a giant elephant, everything has an associated wave with itself, it is a different matter that the wave nature is noticeable or not. He assigned a wavelength to each matter with mass m and momentum p as
通过使光穿过双狭缝观察到的引人注目的干涉图案无疑是光的波动性质的结果。这再次引起了关于光的本质的争论。1704年,牛顿也通过他的微粒理论提出了光的粒子性质。
这两种理论都不足以解释所有与光有关的现象。因此,科学家们开始得出结论,光具有波和粒子性质。1924年,法国物理学家路易斯·德布罗意提出了一个理论。他认为,宇宙中的所有粒子都与波的性质有关,即世界上的任何事物,无论是小光子还是大象,都有与自身相关的波,波的性质是否明显是另一回事。他给每种物质分配了一个波长,质量为m,动量为p
Where, h is Planck constant and p = mv, v is velocity of the body.
Thus due the huge mass of an elephant it has a very significant momentum and hence a very small wavelength, which we are unable to notice. However small particles such as electrons, etc. have very small mass and hence very noticeable wavelength or wave nature. This theory of de Broglie also helps us to explain the discrete existence of orbits in Bohr’s model of atom. An electron will exist in an orbit if its length is equal to integral multiple of its natural wavelength, if it is unable to complete its wavelength then that orbit will not exist.
其中,h是普朗克常数,p=mv,v是物体的速度。
因此,由于大象的巨大质量,它具有非常显著的动量,因此波长非常小,我们无法注意到。然而,诸如电子等小粒子具有非常小的质量,因此具有非常明显的波长或波性质。德布罗意的这一理论也有助于我们解释玻尔原子模型中轨道的离散存在。如果电子的长度等于其自然波长的整数倍,则电子将存在于轨道中,如果电子无法完成其波长,则该轨道将不存在。
Further developments by Davisson and Germer of electron diffraction from a crystal and a similar interference pattern obtained after bombarding a double slit with electrons had strengthen de Broglie’s matter wave theory or the wave particle duality theory.
Davidson和Germer对晶体中电子衍射的进一步发展,以及用电子轰击双缝后获得的类似干涉图,加强了德布罗意的物质波理论或波粒二象性理论。
Compton Effect
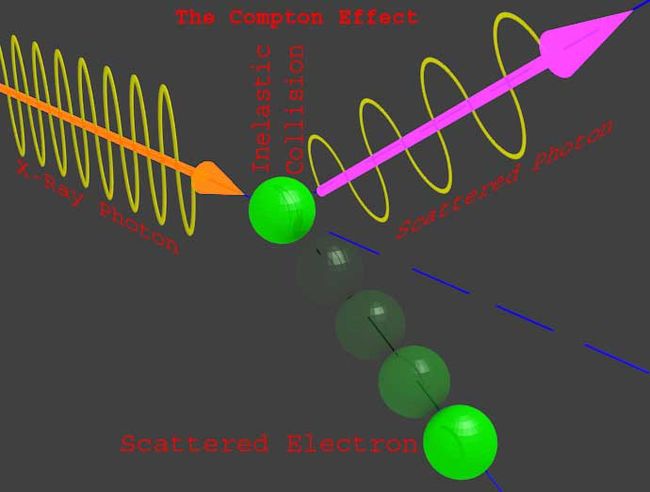
In the photoelectric effect, the light strikes on a metal in the form of beam of particles called photons. The energy of one photon contributes the work function energy of one electron as well as provides the kinetic energy to that emitted electron. These photons are the particle like behavior of light wave. Sir Albert Einstein proposed that light is the collective effect of huge number of energy packets called photon where each photon contains energy of hf. Where h is the Planck constant and f is the frequency of the light. This is a particle like behavior of light wave. The particle like behavior of light-wave or other electromagnetic wave can be explained by Compton effect.
In this experiment, one x ray beam of frequency fo and wavelength λo was incident on an electron. After hitting the electron by incident x-ray it is found that the electron and incident x-ray both are scattered into two different angles with respect to the axis of incident x-ray. This collision obeys the energy conversation principle just like collision of Newtonian’s particles. It was found that after the collision the electron gets accelerated in a particular direction and the incident x-ray is diffracted in another direction and it was also observed that diffracted ray has a different frequency and wavelength than the incident x-ray. As the energy of the photon varies with frequency it can be concluded that the incident x-ray losses an energy during collisions and the frequency of the diffracted ray is always less than that of the incident x-ray. This lost energy of x-ray photon contributes the kinetic energy for the movement of the electron. This collision of x-ray or its photon and electron is just like to Newtonian’s particles such as Billboard balls.
The energy of photon is given by
康普顿效应
在光电效应中,光以称为光子的粒子束的形式照射在金属上。一个光子的能量贡献了一个电子的功函数能量,并为发射的电子提供动能。这些光子是光波的粒子状行为。阿尔伯特·爱因斯坦爵士提出,光是大量称为光子的能量包的集体效应,其中每个光子包含hf的能量,其中h是普朗克常数,f是光的频率。这是光波的粒子状行为。
光波或其他电磁波的粒子状行为可以用康普顿效应来解释。在本实验中,一个频率为fo、波长为λo的x射线束入射到电子上。在入射x射线撞击电子后,发现电子和入射x射线都相对于入射x射线的轴散射成两个不同的角度。这种碰撞遵循能量守恒原理,就像牛顿粒子的碰撞一样。发现碰撞后电子在特定方向上加速,入射x射线在另一方向上衍射,还观察到衍射射线具有与入射x射线不同的频率和波长。由于光子的能量随频率变化,可以得出结论,入射x射线在碰撞期间损失能量,并且衍射射线的频率总是小于入射x射线的频率。x射线光子的能量损失为电子的运动提供了动能。x射线或其光子与电子的碰撞就像是牛顿的粒子,比如广告牌球。
光子的能量由下式给出:
Therefore the momentum of the photon can be proved as
因此,光子的动量可以证明为:
Which can be written as,
From equation (1) it can be concluded that a electromagnetic wave with wavelength λ will have the photon with momentum p.
From equation (2) it can be concluded that a particle with momentum p is associated with wavelength λ. That means wave has particle like characteristics, the particle on movement also exhibits wave like behaviour.
从方程(1)可以得出结论,波长λ的电磁波将具有动量p的光子。
从方程(2)可以得出结论,动量为p的粒子与波长λ相关。这意味着波具有类似粒子的特性,运动中的粒子也表现出类似波的行为。
As we already said, this conclusion was first drawn by De Broglie and hence this is known as De Broglie hypothesis. As the wavelength of the moving particle is expressed as
正如我们已经说过的,这个结论是德布罗意首先得出的,因此这被称为德布罗意假说。移动粒子的波长表示为:
Where, p is the momentum, h is Planck constant and wavelength λ is referred as De Broglie’s wavelength. De Broglie explained that as the electrons orbit around the nucleus it will also have the wave like behaviour along with its particle like characteristics.
其中,p为动量,h为普朗克常数,波长λ为德布罗意波长。德布罗意解释说,当电子围绕原子核运行时,它也将具有类似于波的行为以及类似于粒子的特性。
Divission and Germer Experiment
The wave nature of electron can be proved and established in many different ways but most popular experiment is Divission and Germer in the year of 1927. In this experiment they used a beam of accelerated electrons which normally strikes on the surface of a nickel block. They observed the pattern of scattered electrons after striking on the nickel block. They used an electron density monitor for this purpose. Although it was expected that the electron should be scattered after collision in different angle with respect to the axis of the incident electron beam but in the actual experiment it was found that the density of scattered electrons was more at particular angles than other. This angular distribution of the scattered electrons is very similar to an interference that of light diffraction. Hence this experiment clearly shows the existence of wave particle duality of electrons. The same principle can be applied to the proton and neutrons too.
除法与Germer实验
电子的波性质可以用许多不同的方法证明和建立,但最流行的实验是1927年的Division和Germer。在这个实验中,他们使用了一束加速电子,通常撞击镍块表面。他们在撞击镍块后观察到散射电子的图案。为此,他们使用了电子密度监测器。虽然预期电子在碰撞后应以相对于入射电子束轴的不同角度散射,但在实际实验中发现散射电子的密度在特定角度比其他角度更大。散射电子的角分布非常类似于光衍射的干涉。因此,这个实验清楚地表明了电子的波粒二象性的存在。同样的原理也适用于质子和中子。