《Inhibition抑制 of mTORC1 by astrin胃泌素 and stress granules应激颗粒 prevents apoptosis凋亡 in cancer cells》
1. Astrin与mTOR竞争以结合raptor。表现在如果astrin缺失,那么mTORC1会形成得更多。
2. 可以解答为什么我的研究计划中要用到亚硝酸盐Thus, formation of the raptor-astrin complex and its association with SGs is induced by arsenite.
Astrin Is a Specific Raptor Interactor which Inhibits mTORC1 Association
Astrin competes with mTOR for raptor binding, resulting in increased mTORC1 formation in the absence of astrin.
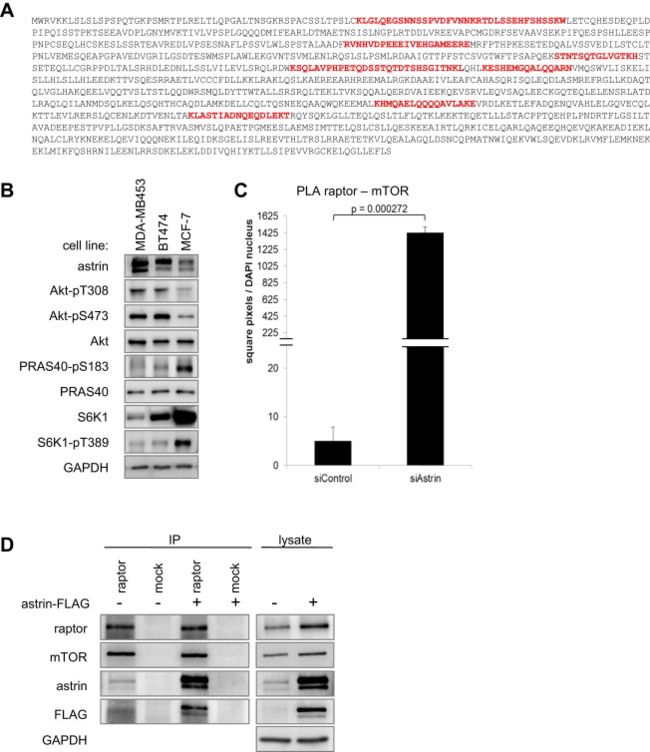
(A) Astrin amino acid sequence. Proteomic analysis of raptor interactors in HeLa cells reveals astrin. Depicted is the astrin full-length sequence. Peptides stretches identified by MS/MS are shown in red.
(B) Astrin expression in breast cancer cell lines correlates with Akt activation, and PRAS40 and S6K1 inhibition. Lysates of the indicated breast cancer cell lines were detected by IB.
Astrin protein levels positively correlated with Akt activity, and negatively correlated with phosphorylation of the mTORC1 substrates PRAS40-S183 and S6K1-T389 (Figure S1B); raising the question whether astrin exerts an inhibitory effect on mTORC1.
(C) Signal intensity quantitation of mTOR-raptor PLA (Figure 1G). This quantitation method yields a comparable result as the counted dots per cell (shown in Figure 1H). Data represented as mean ± SEM.
(D) mTOR-raptor co-IP in the presence of overexpressed astrin-FLAG. HeLa cells were transfected with astrin-FLAG; IPs were done with raptor or control antibodies, and detected by IB. This co-IP has been performed in parallel with the data shown in Figure 1A.
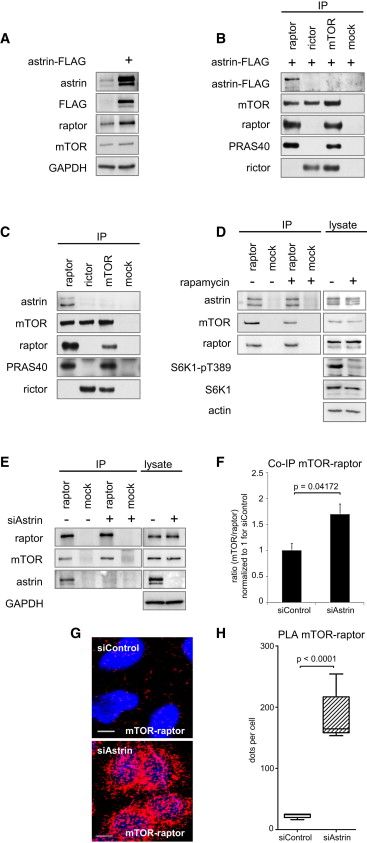
(A) Astrin-FLAG overexpression induces raptor levels.
(B) Astrin-FLAG coimmunoprecipitates免疫共沉淀 with raptor, but not with mTOR or rictor.
(C) Endogenous 内源性astrin coimmunoprecipitates with raptor, but not with mTOR or rictor.
(D) Rapamycin does not alter raptor-astrin binding.
(E) Astrin siRNA induces mTOR-raptor co-IP.
(F) Relative quantitation of mTOR co-IP with raptor (E). Ratio mTOR/raptor for n = 5 experiments. Data normalized to 1 for siControl, and represented as mean ± SEM.
(G) Astrin siRNA induces mTOR-raptor association in situ原地. Nuclei 原子核were stained with DAPI (blue), and PLA was performed for raptor and mTOR (red).
(H) Relative quantitation of mTORC1 assembly in situ without and with astrin siRNA (G). Dots/cell on control and astrin siRNA slides. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers). See Figure S1C for quantitation of fluorescence intensity.
Experiments performed in HeLa cells. Data representative of at least three independent experiments. See also Figure S1.
Astrin Inhibits mTORC1 Signaling
- astrin deficiency in nonsynchronized cells induces phosphorylation of the mTORC1 substrate S6K1-T389, and this effect is independent of astrin’s mitotic functions.
- overall mTORC1 network dynamics are accelerated in the absence of astrin, and this correlates with an increase in mTOR-raptor association.
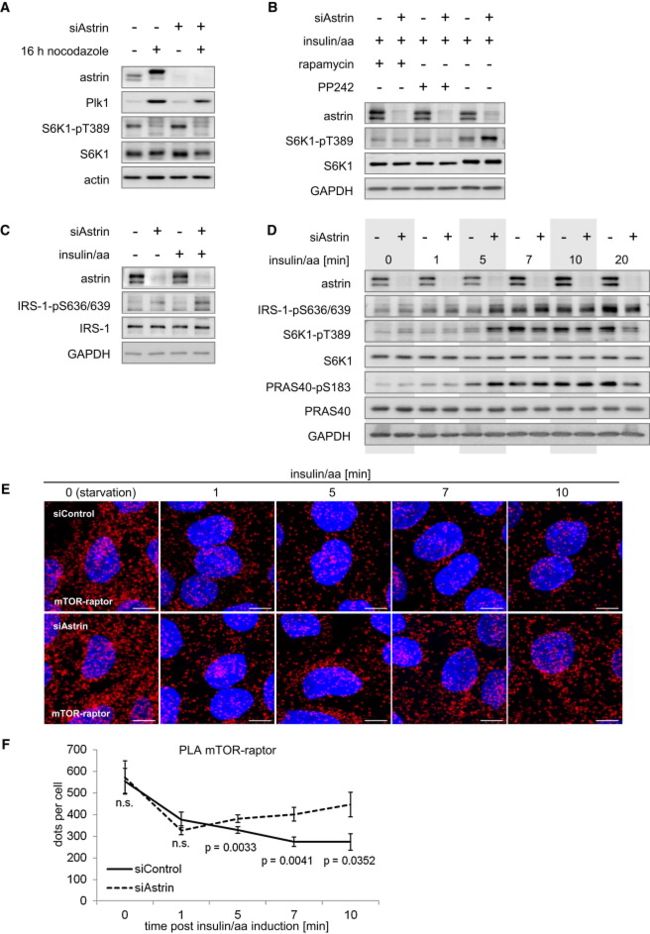
(A) Astrin deficiency does not alter S6K1 phosphorylation in mitotic cells分裂细胞 but induces p70-S6K1-pT389 in nonsynchronized 非同步cultures. HeLa cells were synchronized同步 at G2/M, or left nonsynchronized左非同步. See Figure S2A for relative quantitation of S6K1-T389 without and with siAstrin under basal conditions.
(B) Astrin-deficiency-induced S6K1 phosphorylation is inhibited by the mTOR inhibitors rapamycin and PP242. HeLa cells serum/aa starved; treated with PP242, rapamycin, or carrier (DMSO); and induced with insulin/aa.
(C) Astrin deficiency induces IRS-1 phosphorylation. Treatment as in Figure 2B.
(D) The dynamic of mTORC1 (S6K1 phosphorylation) induction and NFL activation (IRS-1 phosphorylation) is accelerated by astrin deficiency. Treatment as in Figure 2B. See Figure S2A for relative quantitation of S6K1-T389.
(E) Astrin deficiency induces mTOR-raptor association in response to insulin/aa. Treatment as in Figure 2B. Nuclei stained with DAPI (blue); PLA performed for raptor and mTOR (red).
(F) Relative quantitation of mTORC1 assembly in situ in response to insulin/aa without and with astrin siRNA (E). Dots/cell were counted. Data represented as mean ± SEM.
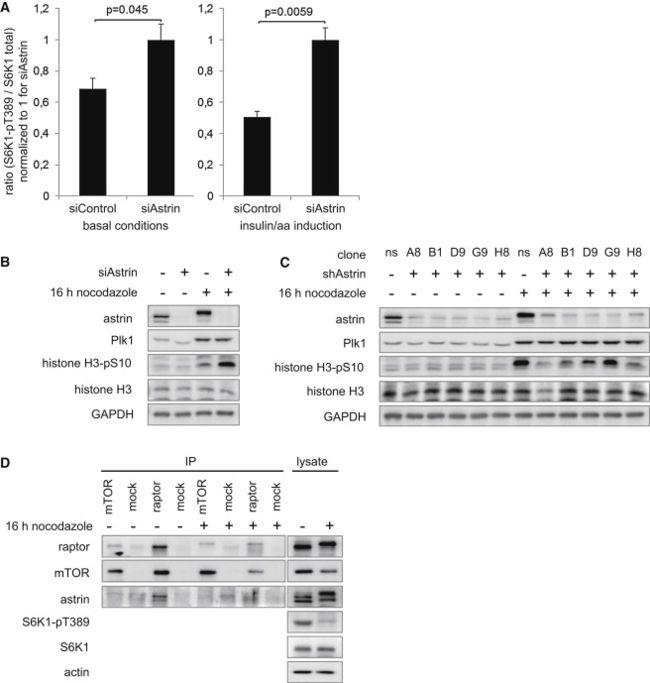
(A) Relative quantitation of S6K1-pT389 upon astrin knockdown under basal conditions基础状态 (left, related to Figure 2A) and insulin/amino acid induction (right, related to Figures 2B and 2D). n = 3; data represented as mean ± SEM.
(B) Astrin siRNA does not induce mitotic markers in nonsynchronized cells. HeLa cells were transfected with control or astrin siRNA; arrested with nocodazole at G/2M or left unsynchronized; and analyzed by IB;
histone-H3-pS10, Plk1 = mitosis markers.
(C) Astrin shRNA does not induce mitotic markers in nonsynchronized cells. HeLa cells stably transduced with different inducible shRNA sequences against astrin were cultivated under control conditions or knockdown induction with doxycycline多四环素; arrested with nocodazole诺考达唑 at G/2M or left unsynchronized; and analyzed by IB;
histone-H3-pS10, Plk1 = mitosis markers; ns, nonsilencing; character-number designations, different clones with different shRNA sequences against astrin.
(D) raptor-astrin binding is ablated脱落 in mitotic cells. HeLa cells were synchronized 同步at G2/M with nocodazole and released for 30 min, or left nonsynchronized; IPs were done with raptor, mTOR, and control antibodies and detected by IB.
The Astrin-Raptor Complex Localizes to SGs
- astrin’s N-terminal head domain mediates primarily raptor binding, whereas astrin’s C-terminal coiled-coil domains mediate mainly its localization.
- upon arsenite, astrin and raptor, but not mTOR, colocalize with SGs.
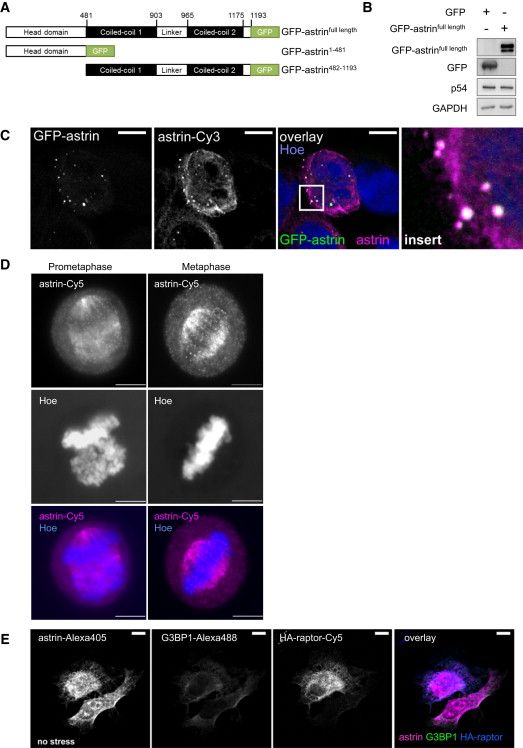
(A) Scheme of GFP-astrin fusion constructs. Similar constructs were made for N-terminal GFP fusions. Results with N- and C-terminal GFP-astrin fusion proteins were similar.
(B) GFP-astrin and p54/DDX6 expression. IB analysis of cells in Figures 3D and 3E.
(C) Astrin antibody specifically detects GFP-astrin. Cells were transfected with GFP-astrinfull length, stained with commercial astrin antibody (Santa Cruz), and Hoechst, and detected by confocal microscopy.
Left: GFP-astrin;
2nd image: commercial astrin antibody staining;
3rd image: overlay覆盖 (white) of GFP-astrinfull length (green), commercial astrin antibody (magenta品红), and Hoechst (blue);
4th image: Insert: blow-up放大 of square in 3rd image.
(D) Astrin antibody specifically detects astrin at the mitotic spindle有丝分裂纺锤体. HeLa cells were synchronized同步 at G2/M with nocodazole and released for 60 min; stained with commercial astrin antibody (Santa Cruz), and Hoechst; and detected by fluorescence microscopy; cells in prometaphase前中期 and metaphase中期 are shown.
(E) In unstressed cells, HA-raptor, astrin, and G3BP1 do not colocalize in punctuate structures. Cells were transfected with HA-raptor; stained against astrin, G3BP1 and HA, and with Hoechst, and detected by confocal microscopy. Control for Figure 3J.
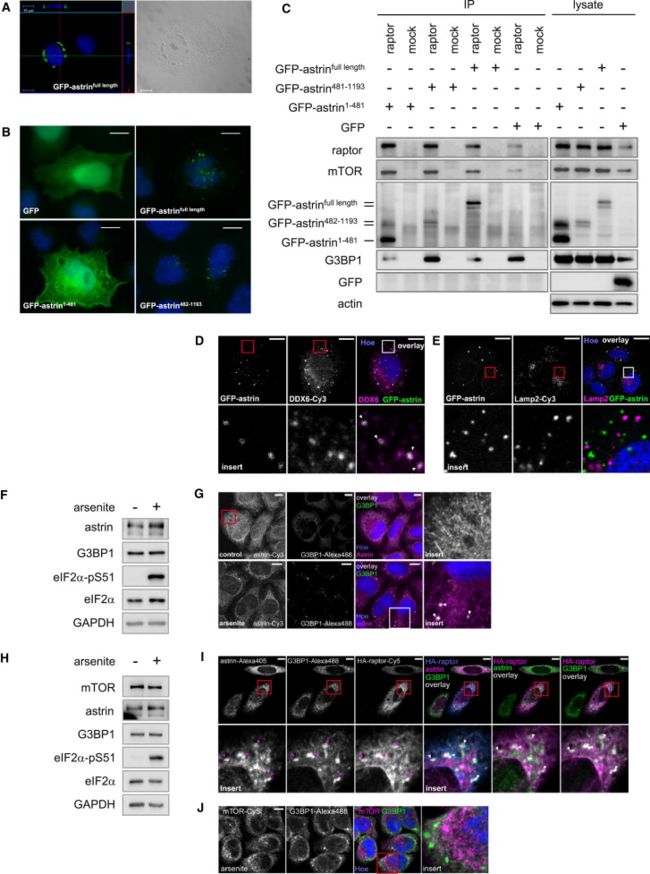
(A) GFP-astrin localizes into granular structures团粒结构 that are visible by light microscopy光学显微镜. GFP-astrinfull length in HeLa cells was detected by confocal microscopy共聚焦显微镜. Left: GFP-astrin (green) and Hoechst (blue). Cross-section through the horizontal and vertical plane. Right: light microscopy of granular 粒状GFP-astrin structures.
(B) The C-terminal coiled-coil 卷曲螺旋domains of astrin mediate调停 its distinct localization. GFP-astrinfull length, GFP-astrin1-481, GFP-astrin482-1193 (green), Hoechst (blue); detection by fluorescence microscopy.
(C) The N-terminal head domain of astrin binds to raptor. Cells expressing GFP-astrinfull length, GFP-astrin1-481, GFP-astrin482-1193, or GFP were used for raptor IPs.
(D) GFP-astrin colocalizes with the SG/PB marker p54/DDX6. GFP-astrin (green), p54/DDX6 (magenta), Hoechst (blue). Insert: blow-up of square. See Figure S3B for IB detection of GFP-astrin and p54/DDX6 expression.
(E) GFP-astrin does not colocalize with the lysosomal marker Lamp2. GFP-astrin (green), Lamp2 (magenta), Hoechst (blue). Insert: blow-up of square. See Figure S3B for IB detection of GFP-astrin expression.
(F) IB analysis of cells in (G) for astrin and G3BP1 expression, and oxidative stress induction (eIF2α-pS51).
(G) Astrin localizes under arsenite stress into G3BP1 positive SGs. HeLa cells cultivated in control and stress conditions (arsenite); astrin (magenta), G3BP1 (green), and Hoechst (blue). White arrows: astrin-G3BP1 colocalization.
(H) IB analysis of cells in (J) for mTOR, astrin, and G3BP1 expression and oxidative stress induction (eIF2α-pS51).
(I) HA-raptor colocalizes with astrin into G3BP1 positive SGs under arsenite stress.
Columns列 1–3: Cells transfected 转染with HA-raptor, arsenite stressed, and stained against 染色对astrin, G3BP1, and HA. Insert: blow-up of square (points of colocalization: magenta arrow heads).
Columns 4–6 (left to right): Triple overlay (white) of HA-raptor (blue), astrin (magenta), G3BP1 (green); double overlay (white) of astrin (green) and HA-raptor (magenta); double overlay (white) of G3BP1 (green) and HA-raptor (magenta). Insert: blow-up of square. See Figure S3E for control in unstressed cells.
(J) mTOR does not colocalize with astrin or G3BP1 under arsenite stress. Arsenite-stressed cells. mTOR (magenta), G3BP1 (green), with Hoechst (blue). Insert: blow-up of square.
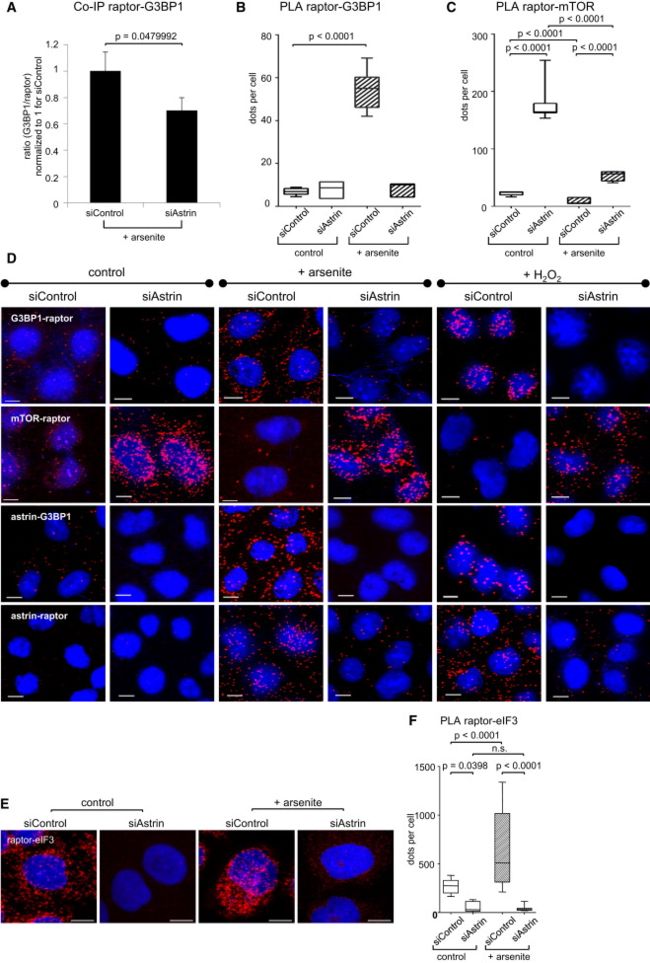
- Oxidative Stress Induces Astrin-Raptor Association in SGs and Reduces Raptor-mTOR association。这可由以下三点佐证:
1) 在arsenite stress 下,raptor binds SG的markerG3BP1,证明Astrin-Raptor定位到了SGs
2) 在arsenite stress 下,astrin-G3BP1 association增强
3) 在arsenite stress 下, astrin association with raptor 增强
2、3证明astrin与 raptor结合并与SGs发生作用,从而竞争性抑制Raptor-mTOR的结合
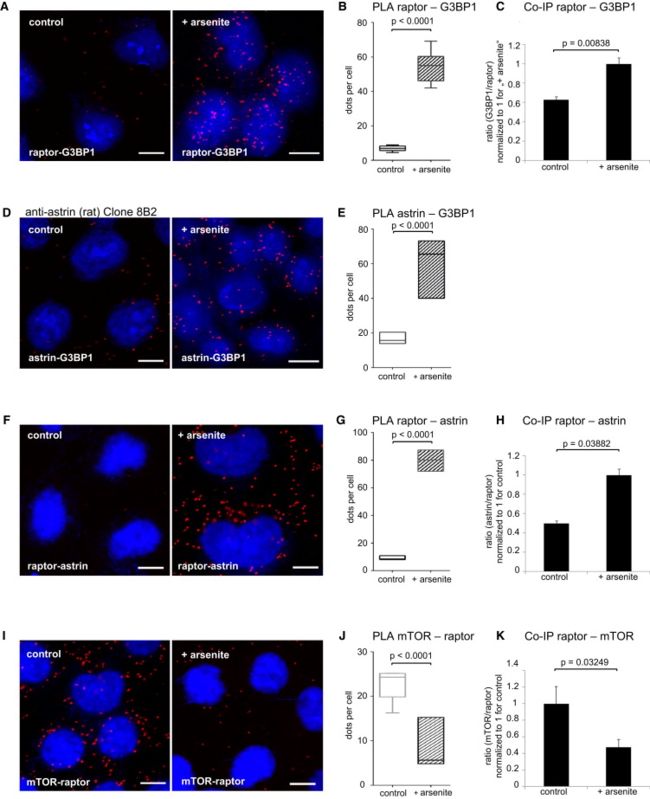
(A) Raptor associates with G3BP1 in situ, and the association is induced by arsenite. DAPI (blue), and PLA for raptor and G3BP1 (red).
(B) Relative quantitation of (A), raptor-G3BP1 association without and with arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile百分率 (whiskers). See Figure S4A for quantitation of fluorescence intensity.
(C) Relative quantitation of coimmunoprecipitated G3BP1 with raptor, without and with arsenite stress. IPs detected by IB (representative example in Figure S4B). G3BP1 signals quantified and normalized to the raptor signal for n = 3 experiments. G3BP1/raptor ratio set to 1 for arsenite treatment. Data represented as mean ± SEM.
(D) Astrin associates with G3BP1 in situ, and the interaction is induced by arsenite stress. DAPI (blue), PLA with anti-astrin antibody (rat, clone 8B2) and G3BP1 (red). See also Figures S4D and S4E.
(E) Relative quantitation of (D), astrin-G3BP1 association without and with arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers). See Figure S4C for quantitation of fluorescence intensity.
(F) Arsenite stress induces raptor-astrin association in situ. PLA for astrin and raptor (red); DAPI (blue).
(G) Relative quantitation of (F), raptor-astrin association without and with arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers). See Figure S4F for quantitation of fluorescence intensity.
(H) Astrin co-IP with raptor is induced upon arsenite stress. IPs detected by IB (representative example shown in Figure S4G). Astrin signals quantified and normalized to the raptor signal for n = 3 experiments. astrin/raptor ratio set to 1 for arsenite treatment. Data represented as mean ± SEM.
(I) Decreased raptor-mTOR (mTORC1) association in situ upon arsenite stress. PLA for mTOR and raptor (red); DAPI (blue).
(J) Relative quantitation of (I), raptor-mTOR (mTORC1) assembly. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers).
(K) mTOR co-IP with raptor (mTORC1) is reduced upon arsenite stress. IPs detected by IB (representative example shown in Figure S4B). mTOR signals quantified and normalized to the raptor signal for n = 3 experiments. mTOR/raptor ratio set to 1 for control treatment. Data represented as mean ± SEM.
mTOR-raptor interaction is reduced by arsenite stress. Confirmation of PLA result with antibody of different clonality, and representative IB results for Co-IP’s.
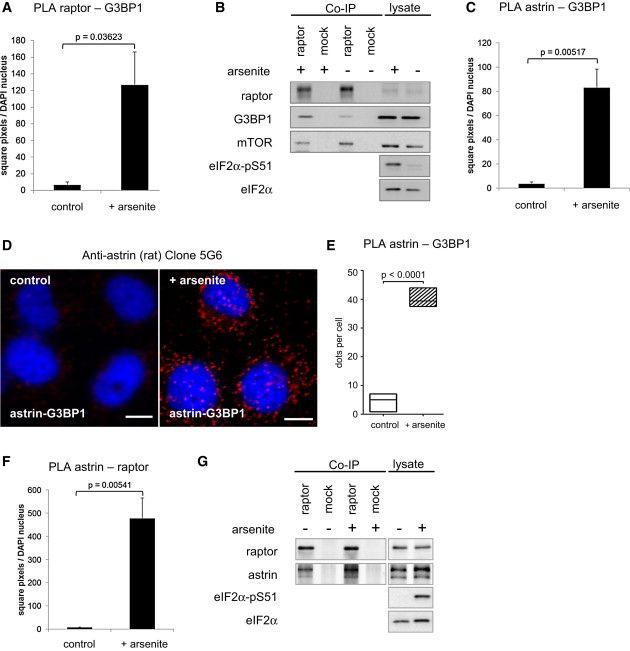
(A) Signal intensity quantitation of raptor-G3BP1 PLA (Figure 4A). This quantitation method yields a comparable result as the counted dots per cell (shown in Figure 4B). Data represented as mean ± SEM.
(B) The SG component G3BP1 coimmunoprecipitates with raptor, and the binding is induced by arsenite stress. In contrast, mTOR-raptor binding is released by arsenite stress. Cells were starved and treated for 30 min with arsenite or carrier. IPs were done with raptor or control antibodies, and detected by IB. Representative example shown for quantitation in Figures 4C and 4K.
(C) Signal intensitiy quantitation of G3BP1-astrin PLA (Figure 4D). This quantitation method yields a comparable result as the counted dots per cell (shown in Figure 4E). Data represented as mean ± SEM.
(D) Independent detection of astrin association with G3BP1 in situ using an astrin antibody of different clonality (rat, clone 5G6). HeLa cells were treated with arsenite or carrier, fixed, and stained with DAPI (blue), and PLA was performed with anti-astrin antibody and G3BP1 (red).
(E) Relative quantitation of (D), astrin-G3BP1 association without and with arsenite stress. Dots/cell were counted on control and arsenite treated slides. Data represented as median (50th percentile), 25th–75th percentile (box), 5th–95th percentile (whiskers).
(F) Signal intensity quantitation of raptor-astrin PLA (Figure 4F). This quantitation method yields a comparable result as the counted dots per cell (shown in Figure 4G). Data represented as mean ± SEM.
(G) astrin co-IP with raptor is induced by arsenite stress. Cells were starved and treated for 30 min with arsenite or carrier载体. IPs were done with raptor or control antibodies, and detected by IB.
Astrin Inhibits mTOR-Raptor Association and Recruits Raptor to SGs
- astrin is required for raptor recruitment to SGs
- 采用 Hydrogen peroxide (H2O2)代替arsenite,可以得到类似的结果
(A) Astrin siRNA reduces raptor-G3BP1 co-IP under arsenite stress. IPs detected by IB (representative example shown in Figure S5A). G3BP1 signals quantified and normalized to the raptor signal for n = 3 experiments. G3BP1/raptor ratio set to 1 for control treatment. Data represented as mean ± SEM.
(B) Relative quantitation of (D), raptor-G3BP1 association without and with astrin siRNA and arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers). See Figure S5B for quantitation of fluorescence intensity.
(C) Relative quantitation of (D), combined arsenite and astrin siRNA effects on mTORC1 assembly (raptor-mTOR). Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers). See Figure S5C for quantitation of fluorescence intensity.
(D) Oxidative stress induced by H2O2 reproduces the effects of arsenite stress on astrin-G3BP1, astrin-raptor, raptor-mTOR, and raptor-G3BP1 complexes without and with astrin siRNA. PLA (red); DAPI (blue).
(E) Astrin mediates raptor binding to the SG marker and translation initiation factor eIF3 in unstressed and arsenite-stressed cells. PLA (red); DAPI (blue).
(F) Relative quantitation of (E), raptor-eIF3 association without and with astrin siRNA and arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers).
(A) The SG component G3BP1 coimmunoprecipitates with raptor, and the binding is induced by siAstrin. Cells were transfected with siAstrin or siControl; starved and treated for 30 min with arsenite or carrier. IPs were done with raptor or control antibodies, and detected by IB. Representative example 典型实例shown for quantitation in Figure 5A.
(B) Signal intensity quantitation of raptor-G3BP1 PLA without and with astrin siRNA and arsenite stress (Figure 5D). This quantitation method yields a comparable result as the counted dots per cell (shown in Figure 5B). Data represented as mean ± SEM.
(C) Signal intensity quantitation of raptor-mTOR PLA without and with astrin siRNA and arsenite stress (Figure 5D). This quantitation method yields a mostly comparable result to the counted dots per cell (shown in Figure 5C); in difference to the dot counts, overall fluorescence shows an induction for mTOR-raptor in siAstrin/arsenite cells as compared to siAstrin/nonstressed cells. This relates to larger dot sizes in siAstrin/arsenite cells. Data represented as mean ± SEM.
(D) PLA Analysis of raptor and raptor-mTOR in nonstimulated cells. HeLa cells were glucose/FCS starved; PLA (red) was performed for raptor with two raptor antibodies from different species recognizing different epitopes (Rabbit, Millipore and mouse, made in house), and for raptor-mTOR. DNA was stained with DAPI (blue).
(E) Relative quantitation of (D), raptor and raptor-mTOR in nonstimulated cells. Dots/cell were counted. Data represented as median (50th percentile), 25th–75th percentile (box), 5th–95th percentile (whiskers).
(F) PLA Analysis of raptor, raptor-astrin, and raptor-mTOR in astrin-deficient and control cells upon arsenite. HeLa cells were treated with arsenite for 30 min; PLA (red) was performed for raptor with two raptor antibodies from different species recognizing different epitopes (Rabbit, Millipore and mouse, made in house), for astrin-raptor, and for raptor-mTOR. DNA was stained with DAPI (blue).
(G) Relative quantitation of (F), raptor in astrin-deficient and control cells upon arsenite. Dots/cell were counted. Data represented as median (50th percentile), 25th–75th percentile (box), 5th–95th percentile (whiskers).
(H) Relative quantitation of (F), raptor-astrin in astrin-deficient and control cells upon arsenite. Dots/cell were counted. Data represented as median (50th percentile), 25th–75th percentile (box), 5th–95th percentile (whiskers).
(I) Relative quantitation of (F), mTOR-raptor in astrin-deficient and control cells upon arsenite. Dots/cell were counted. Data represented as median (50th percentile), 25th–75th percentile (box), 5th–95th percentile (whiskers).
Astrin-Mediated Inhibition of mTORC1 under Stress Is SG Dependent
- SG formation did not require astrin or mTORC1
- 因为astrin inhibition induces mTORC1 upon oxidative stress.
- 所以可以推断astrin prevents mTORC1 hyperactivation under conditions that induce SGs. eg. heat shock、Arsenite
- mTORC1 under stress may be active at a site other than lysosomes溶酶体, raising the possibility of a novel yet unknown mechanism of mTORC1 activation.
(A) Astrin siRNA does not prevent SG assembly under oxidative stress. G3BP1 (white), Hoechst (blue).
(B) IB analysis of (A) for astrin siRNA efficiency, G3BP1 expression, and oxidative stress induction (eIF2α-pS51).
(C) Raptor shRNA does not prevent SG assembly under oxidative stress. G3BP1 (white); Hoechst (blue).
(D) IB analysis of (C) for raptor shRNA efficiency, G3BP1 expression, and oxidative stress induction (eIF2α-pS51).
(E) Arsenite stress induces phosphorylation of the mTORC1 substrate S6K1-T389, and the effect is inhibited by rapamycin.
(F) Raptor shRNA inhibits phosphorylation of S6K1-T389 under arsenite stress.
(G) Astrin siRNA干扰RNA further induces S6K1-T389 under arsenite stress. See Figure S6A for relative quantitation of S6K1-pT389.
(H) PP242 inhibits induction of S6K1-T389 by arsenite stress and astrin siRNA. See also Figure S6B.
(I) Astrin siRNA induces differential phosphorylation of the mTORC1 substrates S6K1-T389 and 4E-BP1-T37/46 under arsenite stress over time.
(J) Relative quantitation of (K), raptor-Lamp2 association without and with astrin siRNA and arsenite stress. Dots/cell counts. Data represented as median, 25th–75th percentile (box), 5th–95th percentile (whiskers).
(K) Astrin deficiency induces raptor association with the lysosomal marker Lamp2 in unstressed cells, and raptor-Lamp2 association is abolished废除 upon arsenite stress. PLA (red) for Lamp2 and raptor; DAPI (blue).
(A) Relative quantitation of S6K1-pT389 upon astrin knockdown under arsenite (left, related to Figure 6G) and H2O2 induction (right, related to Figure 7D). n = 3; data represented as mean ± SEM.
(B) The mTORC1 inhibitor rapamycin inhibits induction of S6K1-pT389 by arsenite stress and astrin siRNA. Cells were transfected with control or astrin siRNA; treated with rapamycin or carrier; subjected to arsenite stress; and analyzed by IB.
(C) siAstrin induces mTORC1 upon arsenite and heat stress. Cells were transfected with control or astrin siRNA; treated with rapamycin or carrier; subjected to arsenite stress, or heat stress (30 min, 43.5°C); and analyzed by IB.
- SGs Are Required for mTORC1 Inhibition by Astrin under Oxidative Stress, and SG and Astrin-Dependent mTORC1 Repression抑制 Protects Cells from Oxidative Stress-Induced Apoptosis细胞凋亡
(A) Cycloheximide (chx) prevents arsenite-induced SG formation. Fluorescence microscopy, G3BP1 (green), Hoechst (blue).
(B) Chx suppresses the astrin shRNA effect on S6K1-pT389 induction under arsenite stress.
在arsenite stress下,astrin shRNA会增加S6K1-pT389的表达,而加入Chx会使 astrin shRNA的增加作用减弱,因为Chx会抑制SG的形成。
(C) Chx prevents H2O2-induced SG formation. Fluorescence microscopy, G3BP1 (green), Hoechst (blue).
采用H2O2做应激反应剂,得到了相似的结果。
(D) Chx suppresses the astrin shRNA effect on S6K1-pT389 induction under H2O2 stress.
(E) siAstrin sensitizes cells to apoptosis, which is prevented by mTORC1 inhibition. HeLa cells, cleaved PARP = apoptosis marker.
siAstrin增强了cleaved PARP表达,也就是说增强了apoptosis,前面已经证明siAstrin会超活化mTOR1,也就是说siAstrin增强了mTOR1,mTOR1增强了apoptosis,在癌细胞中,Astrin的增多抑制了mTOR1,从而避免了自身细胞的凋亡。但是shRaptor的引入却阻止了siAstrin增强cleaved PARP表达。
(F) siAstrin sensitizes breast cancer cells to apoptosis in the presence but not in the absence of SGs. MCF-7 cells, cleaved PARP = apoptosis marker. See also Figure S7E.
(G) siAstrin sensitizes breast cancer cells to mTORC1-dependent apoptosis. MCF-7 cells; cleaved PARP, cleaved caspase-3 = apoptosis markers.
(H) siAstrin leads to early onset of apoptosis凋亡细胞膜起泡 in the presence but not in the absence of SGs. MCF-7 cells analyzed by live-cell imaging; mean time point of blebbing onset indicated. Data represented as mean ± SEM. See also Movie S1.
siAstrin使得凋亡细胞膜起泡时间变短,但是如果加入CHX抑制了SG形成,siAstrin使得凋亡细胞膜起泡时间变短的这种现象就不再存在。
(I) Early onset of apoptosis in siAstrin cells is suppressed by mTORC1 inhibition (rapamycin). MCF-7 cells analyzed by life cell imaging; mean time point of blebbing onset indicated. Data represented as mean ± SEM. See also Movie S2.
siAstrin使得凋亡细胞膜起泡时间变短,但是如果加入rapamycin抑制了mTORC1,siAstrin使得凋亡细胞膜起泡时间变短的这种现象就不那么明显了。
(J) Schematic representation of astrin- and SG-mediated mTORC1 inhibition and apoptosis suppression.
Astrin suppresses apoptosis in breast cancer cells, and mTORC1 induction by arsenite is PI3K independent. Expression of stress response proteins and of TSC2 upon arsenite stress requires the activity of mTORC1, and TSC2 deficiency induces astrin protein levels.
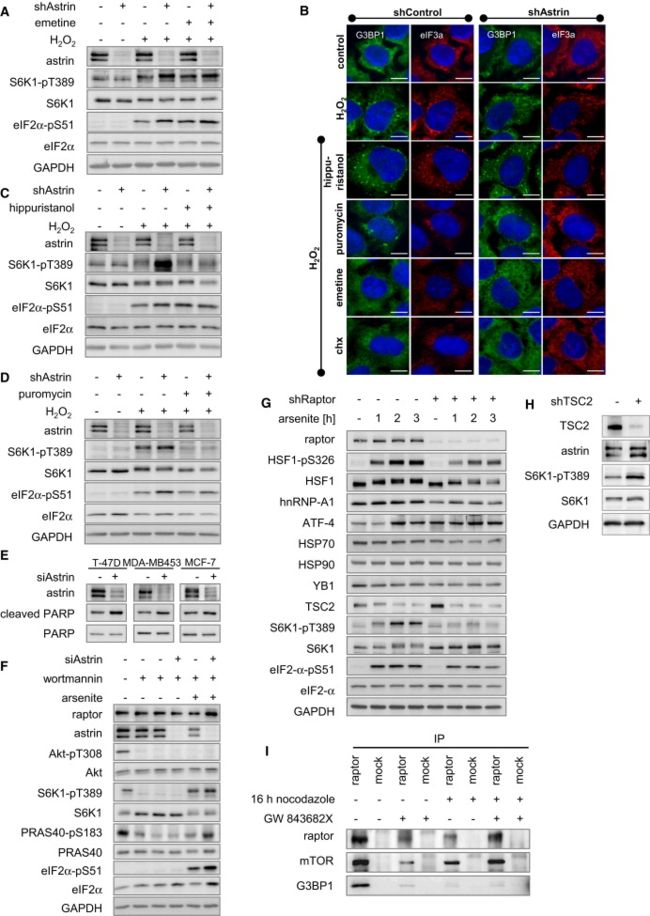
(A) Emetine further induced S6K1-pT389 in shControl cells under H2O2 stress, but not in shAstrin cells. HeLa cells stably transduced with inducible shRNA against astrin (clone-D-9) were cultivated under control or knockdown conditions with doxycycline; pretreated with emetine or carrier; subjected to 30 min H2O2 stress; and analyzed by IB.
(B) Chx and emetine prevent H2O2-induced SG formation, whereas the translational inhibitors puromycin and hippuristanol do not inhibit SGs. HeLa cells stably transduced with inducible shRNA against astrin (clone-D-9) were cultivated under control or knockdown conditions with doxycycline; pretreated with chx, hippuristanol, puromycin, emetine, or carrier; subjected to 30 min H2O2 stress; stained for G3BP1 (green) and with Hoechst (blue); and analyzed by fluorescence microscopy.
emetine和Chx 一样都是抑制SG形成的,所以采用emetine代替Chx做抑制剂,得到了近似的结果,由于emetine和Chx 不仅抑制SG形成,也抑制翻译,所以又采用了 hippuristanol 和puromycin两种不抑制SG形成,只抑制翻译。效果见B、C、D。由于emetine和Chx会使得astrin shRNA增加S6K1-pT389的表达的作用减弱,而 hippuristanol 和puromycin并不会使得astrin shRNA增加S6K1-pT389的表达的作用减弱,因此可以推断emetine和Chx是因为抑制了SG形成而不是抑制了翻译才导致的astrin shRNA增加S6K1-pT389的表达的作用减弱。
(C) Hippuristanol does not induce mTORC1. HeLa cells stably transduced with inducible shRNA against astrin (clone-D-9) were cultivated under control or knockdown conditions with doxycycline; pretreated with hippuristanol or carrier; subjected to 30 min H2O2 stress; and analyzed by IB.
(D) Puromycin does not induce mTORC1. HeLa cells stably transduced with inducible shRNA against astrin (clone-D-9) were cultivated under control or knockdown conditions with doxycycline; pretreated with puromycin or carrier; subjected to 30 min H2O2 stress; and analyzed by IB.
(E) siAstrin facilitates apoptosis in several breast cancer cell lines. The indicated breast cancer cell lines were transfected with control or astrin siRNA; and analyzed by IB; cleaved PARP = apoptosis marker.
(F) Arsenite stress and astrin siRNA induce mTORC1 independently of PI3K and Akt. Cells were transfected with control or astrin siRNA; treated with wortmannin or carrier; subjected to arsenite stress; and analyzed by IB. Phosphorylation of the mTORC1 substrates S6K1-T389, and PRAS40-S183 is induced by oxidative stress and astrin siRNA in the absence and presence of the PI3K inhibitor wortmannin.
(G) Expression of stress response proteins requires mTORC1. HeLa cells stably transduced with inducible shRNA against raptor were cultivated under control or knockdown conditions with doxycycline; subjected to arsenite stress for the indicated times; and analyzed by IB. Several stress related targets (HSF1, hnRNP-A1, ATF-4, HSP70) are regulated in an mTORC1-dependent manner under arsenite stress, whereas others (HSP90, YB1) are not. Thus, mTORC1 differentially controls expression of stress related proteins under arsenite stress. Notably, TSC2 is downregulated by arsenite stress, which correlates with mTORC1-dependent S6K1-pT389 induction in response to arsenite stress.
(H) astrin expession is induced by TSC2 inhibition. HeLa cells stably transduced with inducible shRNA against TSC2 were cultivated under control or knockdown conditions with doxycycline; subjected to arsenite stress; and analyzed by IB. As expected, TSC2 inhibition leads to S6K1-pT389 induction, due to mTORC1 hyperactivation. The astrin protein level is increased when TSC2 is absent.
(I) raptor-G3BP1 binding is ablated in mitotic cells. HeLa cells were synchronized at G2/M with nocodazole and/or the Polo-like-kinase inhibitor GW 843682X, or left nonsynchronized; IPs were done with raptor and control antibodies and detected by IB.
SG and Astrin-Dependent mTORC1 Repression Protects Cancer Cells from Oxidative Stress-Induced Apoptosis
astrin inhibits apoptosis in cancer cells by preventing mTORC1 hyperactivation upon oxidative stress.
应用前景
- astrin inhibition may allow modulation of mTOR network activity specifically in tumor cells, opening new avenues to cancer therapy.