- 博客地址:http://zhwhong.cn/2017/03/27/LIDC-Dicom-data-and-XML-annotation-parse/
- 相关文章:LIDC-IDRI肺结节Dicom数据集解析与总结
- github参考:zhwhong/lidc_nodule_detection
数据来源
数据集采用为 LIDC-IDRI (The Lung Image Database Consortium),该数据集由胸部医学图像文件(如CT、X光片)和对应的诊断结果病变标注组成。该数据是由美国国家癌症研究所(National Cancer Institute)发起收集的,目的是为了研究高危人群早期癌症检测。
该数据集中,共收录了1018个研究实例。对于每个实例中的图像,都由4位经验丰富的胸部放射科医师进行两阶段的诊断标注。在第一阶段,每位医师分别独立诊断并标注病患位置,其中会标注三中类别:1) >=3mm的结节, 2) <3mm的结节, 3) >=3mm的非结节(官网描述: "nodule > or =3 mm," "nodule <3 mm," and "non-nodule > or =3 mm" 详见 Summary)。在随后的第二阶段中,各位医师都分别独立的复审其他三位医师的标注,并给出自己最终的诊断结果。这样的两阶段标注可以在避免forced consensus的前提下,尽可能完整的标注所有结果。
数据位置: @news-ai:/baina/sda1/data/lidc/
解析结果
1.图像矩阵像素信息
模块处理的数据为slicer * rows* cols大小的三维矩阵D。D中第z个切片y行x列的元素对应的位置为:(z rows cols+ y * cols + x) * sizeof(data_type) 。其中rows表示图像的行数,cols表示图像的列数,默认均为512,data_type代表数据类型,默认为short。具体见:肺结节检测说明文档。
- eg: 对于病例LIDC-IDRI-0001,即为133512512的矩阵,一共133张切片,每张大小512*512,依次按顺序存入二进制文件,每个像素大小为2字节(对应C中short类型)。
2.结节区域类型标注信息
第一行: slicers rows cols data_type pixel_space_x pixel_space_y slice_thickness
- slicer : 切片个数;
- rows : 矩阵行数,默认512;
- cols : 矩阵列数,默认512;
- data_type : 数据类型标签。为以下枚举类型中的一种(默认SHORT_TYPE,4):enum DATA_TYPE { CHAR_TYPE, UCHAR_TYPE, INT_TYPE, UINT_TYPE, SHORT_TYPE, USHORT_TYPE, FLOAT_TYPE, DOUBLE_TYPE };
- pixel_space_x : x线列扫描步长,单位:毫米;
- pixel_space_y : x线行扫描步长,单位:毫米;
- slice_thickness : z轴扫描步长(即切片厚度),单位:毫米。
其他行: Type num x1 y1 z1 x2 y2 z2 … xi yi zi ... xn yn zn
Type: "1"表示"nodules", "2"表示"small_nodules","3"表示"non_nodules";
num:该行x,y,z数字的个数(由于一个点有三个坐标,所以num为3的倍数);
Xi, Yi, Zi:该肺结节第i个点的空间坐标,Zi为切片序号;
数据位置: @news-ai:/baina/sda1/data/lidc_matrix/ (DAT为矩阵,TXT为标注)
数据分析
文件结构
目前测试一共1012个病例数据,每个病例文件夹对应结构:
LIDC-IDRI-XXXX / Study Instance UID / Series Instance UID / *.dcm *.xml
- XXXX : 从0000到1012;
- Study Instance UID : 每个病例对应的检查实例号;
- Series Instance UID : 不同检查对应的序列实例号;
- *.dcm ,*.xml : 解析见LIDC-IDRI图像标注处理记录。
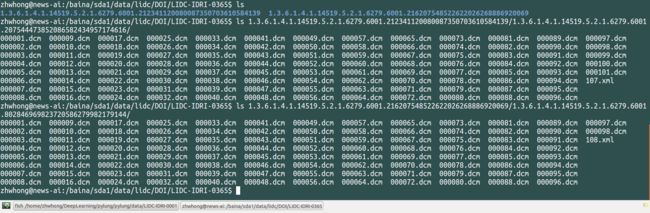
特例:LIDC-IDRI-0365号病例存在两份序列检查,分别有对应的dcm和xml文件,如下:
**Dicom重要信息说明 **
eg : LIDC-IDRI-0001(GE MEDICAL SYSTEM公司)中000001.dcm如下:(详见 DICOM的常用Tag分类和说明)
(0008, 0005) Specific Character Set CS: 'ISO_IR 100'
(0008, 0008) Image Type CS: ['ORIGINAL', 'PRIMARY', 'AXIAL']
(0008, 0016) SOP Class UID UI: CT Image Storage
(0008, 0018) SOP Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.143451261327128179989900675595
(0008, 0020) Study Date DA: '20000101'
(0008, 0021) Series Date DA: '20000101'
(0008, 0022) Acquisition Date DA: '20000101'
(0008, 0023) Content Date DA: '20000101'
(0008, 0024) Overlay Date DA: '20000101'
(0008, 0025) Curve Date DA: '20000101'
(0008, 002a) Acquisition DateTime DT: '20000101'
(0008, 0030) Study Time TM: ''
(0008, 0032) Acquisition Time TM: ''
(0008, 0033) Content Time TM: ''
(0008, 0050) Accession Number SH: '2819497684894126'
(0008, 0060) Modality CS: 'CT'
(0008, 0070) Manufacturer LO: 'GE MEDICAL SYSTEMS'
(0008, 0090) Referring Physician Name PN: ''
(0008, 1090) Manufacturer Model Name LO: 'LightSpeed Plus'
(0008, 1155) Referenced SOP Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.675906998158803995297223798692
(0010, 0010) Patient Name PN: ''
(0010, 0020) Patient ID LO: 'LIDC-IDRI-0001'
(0010, 0030) Patient Birth Date DA: ''
(0010, 0040) Patient Sex CS: ''
(0010, 1010) Patient Age AS: ''
(0010, 21d0) Last Menstrual Date DA: '20000101'
(0012, 0062) Patient Identity Removed CS: 'YES'
(0012, 0063) De-identification Method LO: 'DCM:113100/113105/113107/113108/113109/113111'
(0013, 0010) Private Creator LO: 'CTP'
(0013, 1010) Private tag data LO: 'LIDC-IDRI'
(0013, 1013) Private tag data LO: '62796001'
(0018, 0010) Contrast/Bolus Agent LO: 'IV'
(0018, 0015) Body Part Examined CS: 'CHEST'
(0018, 0022) Scan Options CS: 'HELICAL MODE'
(0018, 0050) Slice Thickness DS: '2.500000'
(0018, 0060) KVP DS: '120'
(0018, 0090) Data Collection Diameter DS: '500.000000'
(0018, 1020) Software Version(s) LO: 'LightSpeedApps2.4.2_H2.4M5'
(0018, 1100) Reconstruction Diameter DS: '360.000000'
(0018, 1110) Distance Source to Detector DS: '949.075012'
(0018, 1111) Distance Source to Patient DS: '541.000000'
(0018, 1120) Gantry/Detector Tilt DS: '0.000000'
(0018, 1130) Table Height DS: '144.399994'
(0018, 1140) Rotation Direction CS: 'CW'
(0018, 1150) Exposure Time IS: '570'
(0018, 1151) X-Ray Tube Current IS: '400'
(0018, 1152) Exposure IS: '4684'
(0018, 1160) Filter Type SH: 'BODY FILTER'
(0018, 1170) Generator Power IS: '48000'
(0018, 1190) Focal Spot(s) DS: '1.200000'
(0018, 1210) Convolution Kernel SH: 'STANDARD'
(0018, 5100) Patient Position CS: 'FFS'
(0020, 000d) Study Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.298806137288633453246975630178
(0020, 000e) Series Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.179049373636438705059720603192
(0020, 0010) Study ID SH: ''
(0020, 0011) Series Number IS: '3000566'
(0020, 0013) Instance Number IS: '80'
(0020, 0032) Image Position (Patient) DS: ['-166.000000', '-171.699997', '-207.500000']
(0020, 0037) Image Orientation (Patient) DS: ['1.000000', '0.000000', '0.000000', '0.000000', '1.000000', '0.000000']
(0020, 0052) Frame of Reference UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.229925374658226729607867499499
(0020, 1040) Position Reference Indicator LO: 'SN'
(0020, 1041) Slice Location DS: '-207.500000'
(0028, 0002) Samples per Pixel US: 1
(0028, 0004) Photometric Interpretation CS: 'MONOCHROME2'
(0028, 0010) Rows US: 512
(0028, 0011) Columns US: 512
(0028, 0030) Pixel Spacing DS: ['0.703125', '0.703125']
(0028, 0100) Bits Allocated US: 16
(0028, 0101) Bits Stored US: 16
(0028, 0102) High Bit US: 15
(0028, 0103) Pixel Representation US: 1
(0028, 0120) Pixel Padding Value US: 63536
(0028, 0303) Longitudinal Temporal Information M CS: 'MODIFIED'
(0028, 1050) Window Center DS: '-600'
(0028, 1051) Window Width DS: '1600'
(0028, 1052) Rescale Intercept DS: '-1024'
(0028, 1053) Rescale Slope DS: '1'
(0038, 0020) Admitting Date DA: '20000101'
(0040, 0002) Scheduled Procedure Step Start Date DA: '20000101'
(0040, 0004) Scheduled Procedure Step End Date DA: '20000101'
(0040, 0244) Performed Procedure Step Start Date DA: '20000101'
(0040, 2016) Placer Order Number / Imaging Servi LO: ''
(0040, 2017) Filler Order Number / Imaging Servi LO: ''
(0040, a075) Verifying Observer Name PN: 'Removed by CTP'
(0040, a123) Person Name PN: 'Removed by CTP'
(0040, a124) UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.335419887712224178340067932923
(0070, 0084) Content Creator's Name PN: ''
(0088, 0140) Storage Media File-set UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.211790042620307056609660772296
(7fe0, 0010) Pixel Data OW: Array of 524288 bytes
eg : LIDC-IDRI-0069(TOSHIBA公司)中000001.dcm如下:
(0008, 0008) Image Type CS: ['ORIGINAL', 'PRIMARY', 'AXIAL']
(0008, 0016) SOP Class UID UI: CT Image Storage
(0008, 0018) SOP Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.263800607656124864093833884216
(0008, 0020) Study Date DA: '20000101'
(0008, 0021) Series Date DA: '20000101'
(0008, 0022) Acquisition Date DA: '20000101'
(0008, 0023) Content Date DA: '20000101'
(0008, 0024) Overlay Date DA: '20000101'
(0008, 0025) Curve Date DA: '20000101'
(0008, 002a) Acquisition DateTime DT: '20000101'
(0008, 0030) Study Time TM: ''
(0008, 0032) Acquisition Time TM: '185549.500'
(0008, 0033) Content Time TM: '185605.277'
(0008, 0050) Accession Number SH: '2819497684894126'
(0008, 0060) Modality CS: 'CT'
(0008, 0070) Manufacturer LO: 'TOSHIBA'
(0008, 0090) Referring Physician Name PN: ''
(0008, 1090) Manufacturer Model Name LO: 'Aquilion'
(0010, 0010) Patient Name PN: ''
(0010, 0020) Patient ID LO: 'LIDC-IDRI-0069'
(0010, 0030) Patient Birth Date DA: ''
(0010, 0040) Patient Sex CS: 'M'
(0010, 1010) Patient Age AS: '051Y'
(0010, 2160) Ethnic Group SH: 'white-ns'
(0010, 21c0) Pregnancy Status US: 4
(0010, 21d0) Last Menstrual Date DA: '20000101'
(0012, 0062) Patient Identity Removed CS: 'YES'
(0012, 0063) De-identification Method LO: 'DCM:113100/113105/113107/113108/113109/113111'
(0013, 0010) Private Creator OB: 'CTP '
(0013, 1010) Private tag data OB: 'LIDC-IDRI '
(0013, 1013) Private tag data OB: '62796001'
(0018, 0010) Contrast/Bolus Agent LO: '100ccs_OMNI-350'
(0018, 0015) Body Part Examined CS: 'CHEST'
(0018, 0022) Scan Options CS: 'HELICAL_CT'
(0018, 0050) Slice Thickness DS: '2.0'
(0018, 0060) KVP DS: '135'
(0018, 0090) Data Collection Diameter DS: '400.00'
(0018, 1020) Software Version(s) LO: 'V2.04ER001'
(0018, 1100) Reconstruction Diameter DS: '379.687'
(0018, 1120) Gantry/Detector Tilt DS: '+0.0'
(0018, 1130) Table Height DS: '+48.00'
(0018, 1140) Rotation Direction CS: 'CW'
(0018, 1150) Exposure Time IS: '500'
(0018, 1151) X-Ray Tube Current IS: '260'
(0018, 1152) Exposure IS: '130'
(0018, 1210) Convolution Kernel SH: 'FC10'
(0018, 5100) Patient Position CS: 'FFS'
(0020, 000d) Study Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.303241414168367763244410429787
(0020, 000e) Series Instance UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.131939324905446238286154504249
(0020, 0010) Study ID SH: ''
(0020, 0011) Series Number IS: '3079'
(0020, 0012) Acquisition Number IS: '5'
(0020, 0013) Instance Number IS: '134'
(0020, 0020) Patient Orientation CS: ['L', 'P']
(0020, 0032) Image Position (Patient) DS: ['-184.375000', '-188.281200', '1292.500000']
(0020, 0037) Image Orientation (Patient) DS: ['1.000000', '0.000000', '0.000000', '0.000000', '1.000000', '0.000000']
(0020, 0052) Frame of Reference UID UI: 1.3.6.1.4.1.14519.5.2.1.6279.6001.228313061349684266844487315959
(0020, 1040) Position Reference Indicator LO: ''
(0020, 1041) Slice Location DS: '+324.00'
(0028, 0002) Samples per Pixel US: 1
(0028, 0004) Photometric Interpretation CS: 'MONOCHROME2'
(0028, 0010) Rows US: 512
(0028, 0011) Columns US: 512
(0028, 0030) Pixel Spacing DS: ['0.741', '0.741']
(0028, 0100) Bits Allocated US: 16
(0028, 0101) Bits Stored US: 16
(0028, 0102) High Bit US: 15
(0028, 0103) Pixel Representation US: 1
(0028, 0303) Longitudinal Temporal Information M CS: 'MODIFIED'
(0028, 1050) Window Center DS: '-500'
(0028, 1051) Window Width DS: '2000'
(0028, 1052) Rescale Intercept DS: '0'
(0028, 1053) Rescale Slope DS: '1'
(0032, 000a) Study Status ID CS: ''
(0032, 1000) Scheduled Study Start Date DA: ''
(0032, 1001) Scheduled Study Start Time TM: ''
(0032, 1060) Requested Procedure Description LO: ''
(0032, 1064) Requested Procedure Code Sequence 1 item(s) ----
(0008, 0104) Code Meaning LO: ''
---------
(0038, 0020) Admitting Date DA: '20000101'
(0040, 0002) Scheduled Procedure Step Start Date DA: '20000101'
(0040, 0003) Scheduled Procedure Step Start Time TM: ''
(0040, 0004) Scheduled Procedure Step End Date DA: '20000101'
(0040, 0005) Scheduled Procedure Step End Time TM: ''
(0040, 0244) Performed Procedure Step Start Date DA: '20000101'
(0040, 0245) Performed Procedure Step Start Time TM: ''
(0040, 2016) Placer Order Number / Imaging Servi LO: ''
(0040, 2017) Filler Order Number / Imaging Servi LO: ''
(0040, a075) Verifying Observer Name PN: 'Removed by CTP'
(0040, a123) Person Name PN: 'Removed by CTP'
(0070, 0084) Content Creator Name PN: ''
(7fe0, 0010) Pixel Data OB or OW: Array of 524288 bytes
可以看到不同公司所做的检查存储信息的格式不太一样,但一些主要信息都还是有的:
- SOP Instance UID 用于唯一区分每一张dcm切片,其中Study Instance UID,Series Instance UID上面已经提过,分别用于区分检查号和一次检查对应序列号。
- Modality 表示检查模态,有MRI,CT,CR,DR等;
- Manufacturer 表示制造商,经分析共有"GE MEDICAL SYSTEMS"(最多), "SIEMENS", "TOSHIBA", "Philips"四家制造商提供数据。详见:/baina/sda1/data/lidc_matrix/information.txt;
- Slice Thickness 表示z方向切片厚度,经统计有GE MEDICAL SYSTEMS:2.50, 1.25,SIEMENS:0.75,1.0, 2.0,3.0,5.0,TOSHIBA:2.0, 3.0, Philips:2.0,1.0,1.5,0.9;
- Instance Number 表示一组切片的序列号,这个可以直接用来将切面排序,在实际CT扫描时,是从胸部靠近头的一侧开始扫描,一次扫描到肺部最下,得到的instance number依次增加,对应的Image Position中的z依次减小,而对应的Slice Location是相对位置,绝大多数情况与Image Positon中的z值相同,依次减小,部分不同公司,如TOSHIBA则Slice Location可能与Image Position中的z不同,由于是相对位置,其Slice Location值为正,并且和Instance Number的变化趋势相同。为了在实际分析是不出现错误,不能仅仅采用Slice Location来对切片进行排序,而应使用Instance Number或者Image Position中的z,此次实验使用的是Instance Number。
- Image Position表示图像的左上角在空间坐标系中的x,y,z坐标,单位是毫米,如果在检查中,则指该序列中第一张影像左上角坐标;
- Slice Location为切片z轴相对位置,单位毫米,大多情况与Image Position中的z相同,但TOSHIBA公司提供的数据里面不同,所以不能仅仅根据这个值来对所有切片进行统一排序;
- Photometric Interpretation:光度计的解释,对于CT图像,用两个枚举值MONOCHROME1,MONOCHROME2.用来判断图像是否是彩色的,MONOCHROME1/2是灰度图,RGB则是真彩色图,还有其他;
- Pixel Spacing 表示像素中心间的物理间距;
- Bits Allocated表示存储每一位像素时分配位数,Bits Stored 表示存储每一位像素所用位数;
- Pixel Representation 表示像素数据的表现类型:这是一个枚举值,分别为十六进制数0000和0001,0000H = 无符号整数,0001H = 2的补码。
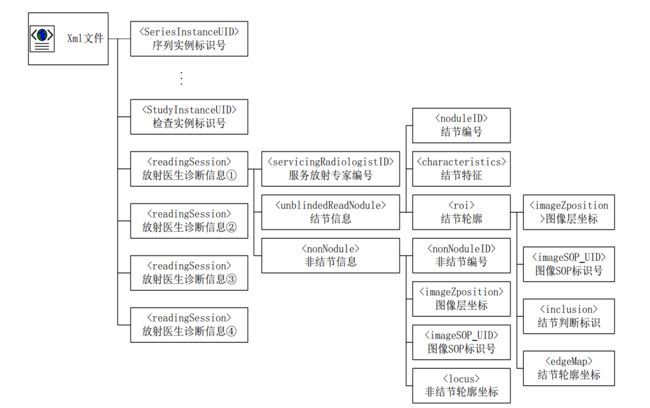
**XML重要信息说明 **
分析所有1012个病人XML标注信息,存在如下问题:
医生标注信息可能有误(个人觉得)!!!!!!
对所有病例跑完标注脚本(/home/zhwhong/API/get_txt.sh)时,在生成的log日志(/baina/sda1/data/lidc_matrix/get_txt.log)里面发现有问题的病例有四个,分别是LIDC-IDRI-0017,LIDC-IDRI-0365,LIDC-IDRI-0566,LIDC-IDRI-0659。
【LIDC-IDRI-0017】
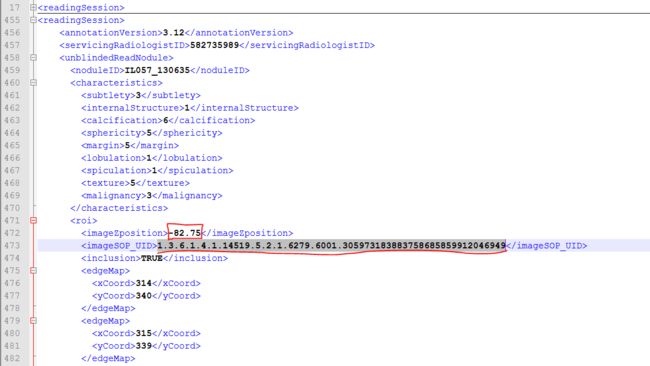
我们找到这个不存在的sop_uid,为"1.3.6.1.4.1.14519.5.2.1.6279.6001.305973183883758685859912046949",然后找到病例17对应的XML文件,看一下医生的标注信息:
带有这个sop_uid的标注有两个,分别是医师2和医师4,我们看一下他们的标注:
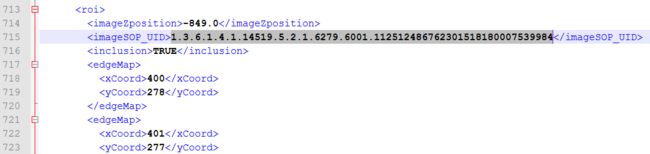
医师2:
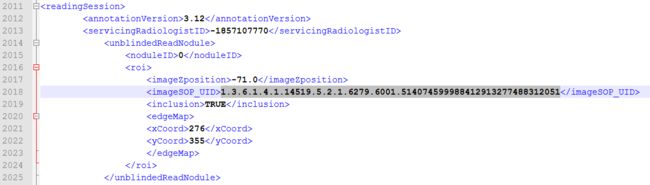
医师4:
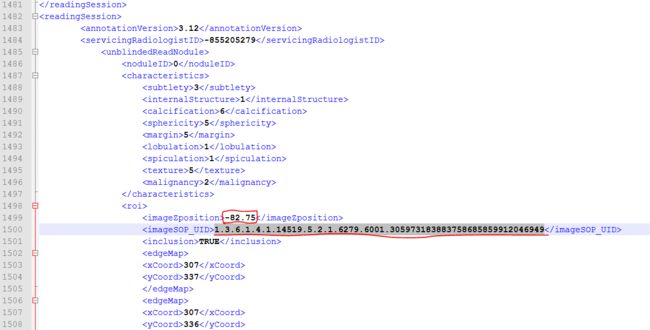
对,有两个医师都标注了这个sop_uid,并且对应的ImageZposition为-82.75,我们再在XML文件中找到ImageZposition为-82.75的另外两个医师是否有标注,结果是有,但是另外两个医师标注的-82.75的位置对应的切片的sop_uid和医师2,4不同,分别如下:
医师1:
医师3:
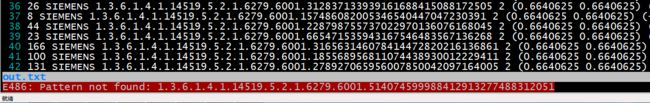
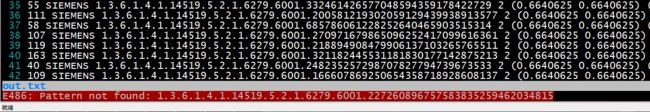
这就很尴尬了,同一个ImageZpositon,但是却标了不同的sop_uid,于是追根溯源,看一下到底是怎么回事,自己写脚本遍历LIDC-IDRI-0017中所有dcm切片,打印出所有切片sop_uid,作对比,然后发现在所有的结果中,根本没有找到医师2,医师4标记的那个sop_uid,而医师1,医师3的标注是存在的,如下:
医师2,4标记的sop_uid找不到:
医师1,3标记的找到了:
所以初步认定,LIDC-IDRI-0017病例中,医师2和医师4存在两处错误的标注信息(sop_uid错误)
【LIDC-IDRI-0365】
LIDC-IDRI-0365中存在两份检查序列,分别是:
1.3.6.1.4.1.14519.5.2.1.6279.6001.212341120080087350703610584139 / 1.3.6.1.4.1.14519.5.2.1.6279.6001.207544473852086582434957174616
和
1.3.6.1.4.1.14519.5.2.1.6279.6001.216207548522622026268886920069 / 1.3.6.1.4.1.14519.5.2.1.6279.6001.802846969823720586279982179144
存在问题的是第二份序列,问题同17号病例类似,如下:
找到医生标注如下(四位医师标注相同):
同样遍历LIDC-IDRI-0365中第二份序列,找不到对应标记的切片sop_uid:
【LIDC-IDRI-0566】
存在和上面相同的问题:
【LIDC-IDRI-0659】
(注:感谢您的阅读,希望本文对您有所帮助。如果觉得不错欢迎分享转载,但请先点击 这里 获取授权。本文由 版权印 提供保护,禁止任何形式的未授权违规转载,谢谢!)