Fibroblast growth factor signalling: from development to cancer
Nicholas Turner* and Richard Grose*
From 《Nature》REVIEWS February 2010|Volume 10
Reporter HXJ / Editor ZQL
Abstract
Fibroblast growth factors (FGFs) and their receptors control a wide range of biological functions, regulating cellular proliferation, survival, migration and differentiation. Although targeting FGF signalling as a cancer therapeutic target has lagged behind that of other receptor tyrosine kinases, there is now substantial evidence for the importance of FGF signalling in the pathogenesis of diverse tumour types, and clinical reagents that specifically target the FGFs or FGF receptors are being developed. Although FGF signalling can drive tumorigenesis, in different contexts FGF signalling can mediate tumour protective functions; the identification of the mechanisms that underlie these differential effects will be important to understand how FGF signalling can be most appropriately therapeutically targeted.
大意:
•FGFs和FGFRs调控非常广泛的生物学功能,如细胞生存、增殖、迁移、分化。
•目前,针对FGF信号通路的肿瘤治疗研究已经落后于其他受体酪氨酸激酶。但是,目前已经有大量证据证明FGFs在各种肿瘤发生过程中的重要性并且靶向FGFs和FGFR的临床药物正在持续发展。
•所以,FGF信号通路的作用机制研究对于理解FGF信号如何恰当地靶向治疗具有重要意义。
FGF信号通路——FGFs和FGFR的结合
a | The basic structure of the fibroblast growth factor (FGF) –FGF receptor (FGFR) complex comprises two receptor molecules, two FGFs and one heparan sulphate proteoglycan (HSPG) chain. The FGF signalling pathway comprises 4 highly conserved transmembrane receptors and 18 FGF ligands (Box 1). FGFs bind with low affinity to cell surface HSPGs (purple) and with high affinity to specific FGFRs. The FGFRs, which are phylogenetically closely related to the vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors (PDGFRs), consist of three extracellular immunoglobulin (Ig) domains, a single transmembrane helix and an intracellular split tyrosine kinase (TK) domain. The second and third Ig domains form the ligand-binding pocket and have distinct domains that bind both FGFs and HSPGs.
成纤维细胞生长因子(FGF)–FGF受体(FGFR)复合物的基本结构包括两个受体分子、两个FGF和一个硫酸肝素蛋白聚糖(HSPG)链。FGF信号通路: 4个高度保守的跨膜受体和18个FGF配体(框1)。FGFs与细胞表面HSPGs(紫色)具有低亲和力,与特异性FGFRs具有高亲和力。在系统学上与血管内皮生长因子受体(VEGFRs)和血小板衍生生长因子受体(PDGFrs)密切相关的FGFRs由三个细胞外免疫球蛋白(IG)结构域、一个跨膜螺旋和一个细胞内分裂酪氨酸激酶(TK)结构域组成。第二和第三个Ig结构域形成配体结合囊,并具有结合FGF和HSPG的独特结构域。
FGFs和FGFRs FGFs:共有23个成员。
FGF配体:其中18个作为FGFR配体, FGF11、FGF12、FGF13和FGF14不作为FGFR配体发挥作用,另外人类不拥有FGF15基因。
激素类FGF:FGF19,FGF21,FGF23FGFR-like 1:无酪氨酸激酶结构域受体
FGF信号通路——配体的特异性结合
b | Ligand-binding specificity is generated by alternative splicing of the Ig III domain. The first half of Ig III is encoded by an invariant exon (IIIa), which is spliced to either exon IIIb or IIIc, both of which splice to the exon that encodes the transmembrane (TM) region. Epithelial tissues predominantly express the IIIb isoform and mesenchymal tissues express IIIc. FGFR4 is expressed as a single isoform that is paralogous to FGFR-IIIc.
b| 配体的特异性结合是由Ig-III域的选择性剪接产生的。Ig III的前半部分由一个固定外显子(IIIa)编码,该外显子拼接到一个外显子IIIb或IIIc,这两个外显子拼接到编码跨膜(TM)区域的外显子。上皮组织主要表达IIIb亚型,间充质组织表达IIIc。FGFR4表达为一个单一的亚型,与FGFR-IIIc相似。
c | Examples of the extent to which ligand specificity can differ between FGFR-IIIb and FGFR-IIIc isoforms, illustrated with the differing ligand specificty of FGFR2 isoforms. The FGFR2-IIIb ligands are shown in blue and the FGFR2-IIIc ligands are shown in brown. For example, FGF7 and FGF10 bind specifically to FGFR2-IIIb and have essentially no binding to FGFR2-IIIc7. The mechanisms controlling splice isoform choice are becoming clearer and defined control elements have been identified in the introns surrounding alternatively spliced exons177,178,179.
c| FGFR-IIIb和FGRR-IIIc亚型之间配体特异性差异程度的示例,以FGFR2亚型的不同配体特异性来说明。FGFR2-IIIb配体呈蓝色,FGFR2-IIIc配体呈棕色。例如,FGF7和FGF10与FGFR2-IIIb特异性结合,并且基本上不与FGFR2-IIIc7结合。控制剪接亚型选择的机制越来越清晰,在选择性剪接外显子177,178,179周围的内含子中已经确定了定义的控制元件。
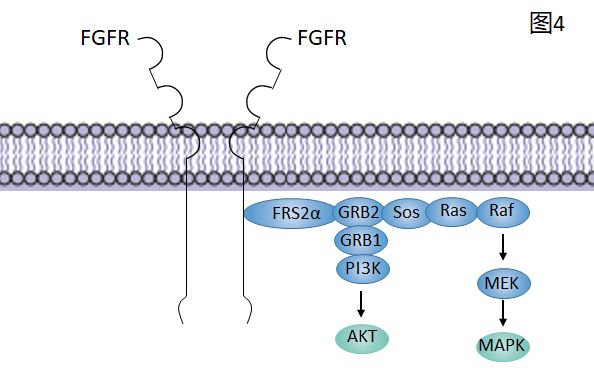
FGF信号通路——下游信号通路
FRS2通过其磷酸酪氨酸(PTB)结构域域结合到FGFRs的近膜区域,然后激活的FGFRs磷酸化FRS2的多个位点使其能够招募调节蛋白GRB2和SOS,最后去激活RAS和其下游的RAF和MAPK通路。
GRB2和GAB1结合招募PI3K结果依赖于AKT的抗凋亡通路,通过酪氨激酶的下游通路
PLCγ在被激活之后,将PIP2水解成PIP3激活细胞内Ga2+通道,从而激活一系列依赖于Ga2+的蛋白。同时,水解DAG激活PKC,然后PKC通过对RAF的磷酸化作用推进MAPK通路。另外,有些蛋白也会被FGFRs激活,但是依赖于细胞环境。如:STATs、p38、MAPK通路、c-Jun、N-terminal kinase(JNK)通路、RSK2DAG, diacylglycerol;
FRS2α FGFR substrate 2α (FGFR底物2α)
GRB2 growth factor receptor-bound 2 (生长因子受体结合2)
Sos son of sevenless
IP3 inositol triphosphate(肌醇三磷酸)
P phosphorylation(磷酸化)
PKC protein kinase C(蛋白激酶C)
PIP2 phosphatidylinositol-4,5-biphosphate(磷脂酰肌醇-4,5-二磷酸)
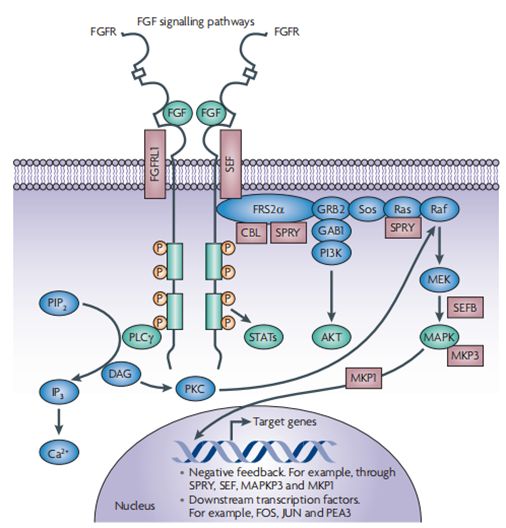
FGFR signalling network: The signal transduction network downstream of fibroblast growth factor (FGF) receptors (FGFRs), along with negative regulators. Following ligand binding and receptor dimerization, the kinase domains transphosphorylate each other, leading to the docking of adaptor proteins and the activation of four key downstream pathways: RAS–RAF–MAPK, PI3K–AKT, signal transducer and activator of transcription (STAT) and phospholipase Cγ (PLCγ) (green).
FGFR信号网络:成纤维细胞生长因子(FGF)受体(FGFRs)下游的信号转导网络,以及负调控因子。在配体结合和受体二聚化之后,激酶域相互磷酸化,导致衔接蛋白的对接和四个关键下游途径的激活:Ras–Raf–MAPK、PI3K–AKT、信号转导和转录激活子(STAT)和磷脂酶Cγ(PLCγ)(绿色)。
FGFRs have also been shown to bind and directly phosphorylate ribosomal S6 kinase17 (not shown). Signalling can be negatively regulated at several levels by receptor internalization or the induction of negative regulators, including FGFR-like 1 (FGFRL1), SEF, Sprouty (SPRY), CBL, MAPK phosphatase 1 (MKP1) and MKP3 (brown). These regulators may modulate ligand binding (FGFRL1 and SEF) or interfere with intracellular signalling, principally through modulation of the MAPK pathway.
FGFRs也被证明能结合并直接磷酸化核糖体S6激酶17(未显示)。信号可通过受体内化或诱导负性调节器在几个水平上负性调节,这些调节器可以调节配体结合(FGFRL1 和 SEF)或干扰细胞内信号传导,主要是通过调节MAPK通路。
FGF信号通路——肿瘤发生过程中的机制
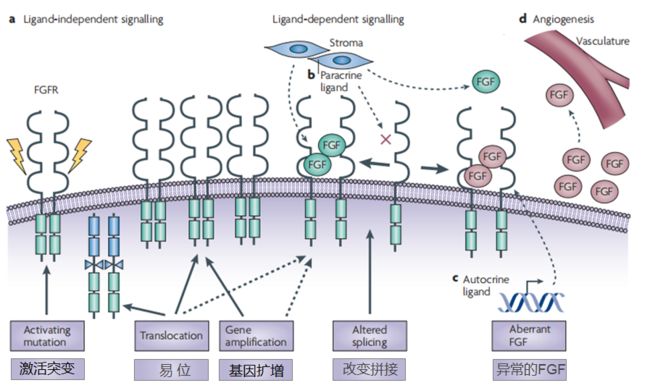
1.FGFRs变异后,在无需配体的情况下自我激活,FGFRs过量表达导致二聚体的自我形成
2.FGFRs拼接的改变导致FGFRs和FGF结合以及FGFRs的过表达使得通路过度的特异性改变,激活。
3.自分泌FGF过表达使得FGF通路过度激活
4.肿瘤细胞自分泌FGF和基质细胞细胞自分泌FGF的过表达促进血管生成
FGFRs和FGF在癌症中的基因改变情况
FGF和FGFR靶向治疗的现状
词汇
activating mutation 激活突变
translocation 易位
gene amplification 基因扩增
altered splicing 改变拼接
aberrant FGF 异常的FGF
paracrine ligand 旁分泌
autocrine ligand 自分泌
angiogenesis [,ændʒɪə(ʊ)'dʒenɪsɪs] 血管生成
vasculature ['væskjələ,tʃʊr] 脉管系统
stroma ['strəʊmə]n. 基质
ligand independent signaling