读《人脑连接组研究:脑结构网络和脑功能网络》
人脑连接组研究:脑结构网络和脑功能网络
doi: 10.1360/972009-2150
文章结构
- 题目
- 人脑连接组研究:脑结构网络和脑功能网络
- 摘要
- 引言
- 1 复杂网络中的一些基本概念
- 1.1 网络的图论描述
- 1.2 “小世界”网络和无标度网络
- 2 脑结构网络
- 2.1 基于磁共振形态学的人脑复杂结构网络
- 2.2 基于磁共振白质纤维束的人脑复杂结构网络
- 2.3 人脑结构网络在脑疾病中的应用研究
- 3 大脑功能网络
- 3.1 基于脑电图和脑磁图的人脑复杂功能网络
- 3.2 基于功能磁共振成像的人脑复杂功能网络
- 3.3 人脑功能网络在脑疾病中的应用研究
- 4 问题与挑战
- 5 总结
- 参考文献
摘要:
人脑是自然界中最复杂的系统之一, 在这个系统中, 多个神经元、神经元集群或者多个脑区 相互连接成庞杂的结构网络, 并通过相互作用完成脑的各种功能. 近年来, 结合基于图论的复杂网 络理论, 研究者们发现利用结构和扩散磁共振成像数据构建的脑结构网络以及利用脑电图/脑磁图 数据和功能磁共振成像数据构建的脑功能网络具有很多重要的拓扑性质, 如“小世界”属性、模块化 的组织结构以及主要分布在联合皮层上的核心脑区(如楔前叶、额上回、额中回). 另一方面, 研究者 发现许多神经精神疾病(如阿尔兹海默病和精神分裂症等)与脑结构和脑功能网络的异常的拓扑变化 有关, 这些研究不仅为理解神经精神疾病的病理机制提供了新视角, 也可能为疾病的早期诊断和治 疗评价提供脑网络影像学标记. 本文以人脑结构和功能连接网络的研究为重点, 介绍了人脑连接组 和复杂网络理论的基本概念, 并且回顾了近年来人脑结构和功能连接组的研究成果, 并指出了该领 域中存在的问题及未来的研究方向.
关键词
脑网络 脑连接 磁共振 图论 小世界 人脑是自然界中最复杂的系统之一.
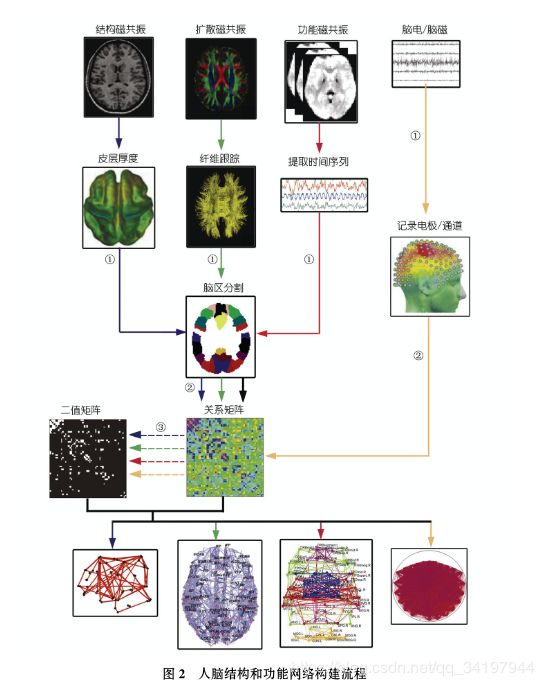
图
人脑结构网络的构建可以分别基于结构磁共振图像(灰质的形态学指标, 如皮层厚度、皮层曲面积等; 蓝色箭头所示流程)和扩散磁共振图像 (白质纤维束, 绿色箭头所示流程); 人脑功能网络可以分别基于功能磁共振图像(大脑功能活动的时间序列, 红色箭头所示流程)和脑电/脑磁信 号(黄色箭头所示流程).
- 网络节点定义: 结构、扩散和功能磁共振数据需要利用先验图谱划分脑区或图像体素定义网络节点, 而脑电/脑磁 数据则直接以记录电极/通道为网络节点.
- 网络连接(边)定义: 基于结构磁共振数据的网络连接定义为网络节点形态学指标之间的统计关 系, 扩散磁共振数据通过确定性或概率性纤维跟踪技术确定网络节点之间的解剖连接, 基于功能磁共振及脑电/脑磁的网络连接一般可以通过 皮尔森相关、偏相关、同步似然性等计算方法度量网络节点的神经活动信号之间的统计关系.
- 构建人脑结构和功能网络: 可以对第 3 步得 到的相关矩阵进行二值化, 获得不同阈值下的二值矩阵, 即大脑结构和功能网络节点主要分布在大脑顶叶,
表
参考文献
1 Sporns O, Tononi G, Kotter R. The human connectome: A structural description of the human brain. PLoS Comput Biol, 2005, 1: 42
2 Lichtman J W, Sanes J R. Ome sweet ome: What can the genome tell us about the connectome? Curr Opin Neurobiol, 2008, 18: 346—353
3 Lichtman J W, Livet J, Sanes J R. A technicolour approach to the connectome. Nat Rev Neurosci, 2008, 9: 417—422
4 Varela, Lachaux F, Rodriguez J P, et al. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci, 2001, 2: 229 —239
5 Crick F, Jones E. Backwardness of human neuroanatomy. Nature, 1993, 361: 109—110
6 Lehrer J. Neuroscience: Making connections. Nature, 2009, 457: 524—527
7 Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 2009, 10: 186—198
8 Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett, 2001, 87: 198701
9 Boccaletti S, Latora V, Moreno Y, et al. Complex networks: Structure and dynamics. Phys Rep, 2006, 424: 175—308
10 Watts D J, Strogatz S H. Collective dynamics of ‘small-world’ networks. Nature, 1998, 393: 440—442
11 Humphries M D, Gurney K, Prescott T J. The brainstem reticular formation is a small world, not scale-free, network. Proc R Soc Lond B Biol Sci, 2006, 273: 503—511
12 Barabasi A L, Albert R. Emergence of scaling in random networks. Science, 1999, 286: 509—512
13 White J G, Southgate E, Thomson J N, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci, 1986, 314: 1—340
14 Felleman D J, Van Essen D C. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex, 1991, 1: 1—47
15 Young M P, Scannell J W, O’Neill M A, et al. Non-metric multidimensional scaling in the analysis of neuroanatomical connection data and the organization of the primate cortical visual system. Philos Trans R Soc Lond B Biol Sci, 1995. 348: 281—308
16 Young M P. Objective analysis of the topological organization of the primate cortical visual system. Nature, 1992, 358: 152—155
17 Young M P. The organization of neural systems in the primate cerebral cortex. Proc Biol Sci, 1993, 252: 13—18
18 Scannell J W, Burns G A, Hilgetag C C, et al. The connectional organization of the cortico-thalamic system of the cat. Cereb Cortex, 1999, 9: 277—299
19 Hilgetag C C, Burns G A, O’Neill M A, et al. Anatomical connectivity defines the organization of clusters of cortical areas in the macaque monkey and the cat. Philos Trans R Soc Lond B Biol Sci, 2000. 355: 91—110
20 Sporns O, Zwi J D. The small world of the cerebral cortex. Neuroinformatics, 2004, 2: 145—162
21 He Y, Chen Z J, Gong G L, et al. Neuronal networks in Alzheimer’s disease. Neuroscientist, 2009, 15: 333—350
22 Mechelli A, Friston K J, Frackowiak R S, et al. Structural covariance in the human cortex. J Neurosci, 2005,
25: 8303—8310 23 Lerch J P, Worsley K, Shaw W P, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage, 2006, 31: 993—1003
24 Thompson P M, Cannon T D, Narr K L, et al. Genetic influences on brain structure. Nat Neurosci, 2001, 4: 1253—1258 25 Mechelli A, Crinion J T, Noppeney U, et al. Neurolinguistics: Structural plasticity in the bilingual brain. Nature, 2004, 431: 757
26 He Y, Chen Z, Evans A C. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex, 2007, 17: 2407—2419
27 Chen Z, He Y, Rosa-Neto P, et al. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex, 2008, 18: 2374—2381
28 Schmitt J E, Lenroot R K, Wallace G L, et al. Identification of genetically mediated cortical networks: A multivariate study of pediatric twins and siblings. Cereb Cortex, 2008, 18: 1737—1747
29 Lenroot R K, Bassett D S, Clasen L S, et al. Developmental changes in topographic properties of anatomical networks in children and adolescents. NeuroImage, 2009, 47: S175
30 Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci, 2003, 4: 469—480
31 Mori S, van Zijl P C. Fiber tracking: Principles and strategies—a technical review. NMR Biomed, 2002, 15: 468—480
32 Hagmann P, Kurant M, Gigandet X, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE, 2007, 2: e597
33 Iturria-Medina Y, Sotero R C, Canales-Rodriguez E J, et al. Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. Neuroimage, 2008, 40: 1064—1076
34 Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic ana- tomical parcellation of the MNI MRI single-subject brain. Neuroimage, 2002, 15: 273—289
35 Gong G, He Y, Concha L, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex, 2008, 19: 524—536
36 Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of human cerebral cortex. PLoS Biol, 2008, 6: e159
37 Li Y, Liu Y, Li J, et al. Brain anatomical network and intelligence. PLoS Comput Biol, 2009, 5: e1000395
38 Yan C G, Gong G L, Wang J H, et al. Anatomical connectivity patterns of human cerebral cortex are associated with brain size, sex and intelligence. NeuroImage, 2009, 47: S128
39 Gong G, Rosa-Neto P, Carbonell F, et al. Age- and gender-related differences in the cortical anatomical network. J Neurosci, 2009, 29: 15684—15693
40 He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci, 2008, 28: 4756—4766
41 Bassett D S, Bullmore E, Verchinski B A, et al. Hierarchical organization of human cortical networks in health and schizophrenia. J Neu- rosci, 2008, 28: 9239—9248
42 He Y, Dagher A, Chen Z, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain, 2009, 132: 3366—3379
43 Cammoun L, Gigandet X, Sporns O, et al. Connectome alterations in schizophrenia. NeuroImage, 2009, 47: S157
44 Vaessen M J, Jansen J F, Hofman P A, et al. Impaired small-world structural brain networks in chronic epilepsy. NeuroImage, 200
45 Friston K J, Frith C D, Liddle P F, et al. Functional connectivity: The principal component analysis of large (PET) data sets. J Cereb Blood Flow Metab, 1993, 13: 5—14
46 Stam C J. From synchronization to networks: Assessment of functional connectivity in the brain. In: Perez Velazquez J L, Richard W, eds. Coordinated Activity in the Brain, vol 2. Berlin Heidelberg: Springer-Verlag, 2009. 91—115
47 Stephan, Hilgetag K E, Burns C C, et al. Computational analysis of functional connectivity between areas of primate cerebral cortex. Philos Trans R Soc Lond B Biol Sci, 2000, 355: 111—126
48 Micheloyannis S, Pachou S, Stam C J, et al. Using graph theoretical analysis of multi channel EEG to evaluate the neural efficiency hy- pothesis. Neurosci Lett, 2006, 402: 273—277
49 Micheloyannis S, Vourkas S, Tsirka M, et al. The influence of ageing on complex brain networks: A graph theoretical analysis. Hum Brain Mapp, 2009, 30: 200—208
50 Ferri R, Rundo F, Bruni O, et al. Small-world network organization of functional connectivity of EEG slow-wave activity during sleep. Clin Neurophysiol, 2007, 118: 449—456
51 Dimitriadis S I, Laskaris N A, Del Rio-Portilla Y, et al. Characterizing dynamic functional connectivity across sleep stages from EEG. Brain Topogr, 2009, 22: 119—133
52 Stam C J. Functional connectivity patterns of human magnetoencephalographic recordings: A ‘small-world’ network? Neurosci Lett, 2004, 355: 25—28
53 Bassett D S, Meyer-Lindenberg A, Achard S, et al. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA, 2006, 103: 19518—19523
54 Ogawa S. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA, 1990, 87: 9868— 9872
55 Biswal B, Zerrin Yetkin F, Haughton V, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med, 1995, 34: 537—541
56 Fox M D, Raichle M E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neuro- sci, 2007, 8: 700—711
57 Salvador R, Suckling J, Coleman M R, et al. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex, 2005, 15: 1332—1342
58 Achard S, Salvador R, Whitcher B, et al. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci, 2006, 26: 63—72
59 Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol, 2007, 3: e17
60 Meunier D, Achard S, Morcom A, et al. Age-related changes in modular organization of human brain functional networks. NeuroImage, 2009, 44: 715—723
61 He Y, Wang J H, Wang L, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One, 2009, 4: e5226
62 Wang J H, Wang L, Zang Y F, et al. Parcellation-dependent small-world brain functional networks: A resting-state fMRI study. Hum Brain Mapp, 2009, 30: 1511—1523
63 Eguíluz V M, Chialvo D R, Cecchi G A, et al. Scale-free brain functional networks. Phys Rev Lett, 2005, 94: 018102
64 van den Heuvel M P, Stam C J, Boersma M, et al. Small-world and scale-free organization of voxel-based resting-state functional connec- tivity in the human brain. Neuroimage, 2008, 43: 528—539
65 Laurienti P J. Modularity maps reveal community structure in the resting human brain. Nat Preced, 2009, hdl:10101/npre.2009.3069.1
66 van den Heuvel M P, Stam C J, Kahn R S, et al. Efficiency of functional brain networks and intellectual performance. J Neurosci, 2009, 29: 7619—7624
67 Delbeuck X, Van der Linden M, Collette F, Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev, 2003, 13: 79—92
68 Friston K J. The disconnection hypothesis. Schizophr Res, 1998, 30: 115—125
69 Stam C J, Jones B F, Nolte G, et al. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex, 2007, 17: 92 —99
70 Stam C J, de Haan W, Daffertshofer A, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alz- heimer’s disease. Brain, 2009, 132: 213—224
71 Supekar K, Menon V, Rubin D, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol, 2008, 4: e1000100
72Buckner R L, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci, 2009, 29: 1860—1873
73 Micheloyannis S, Pachou E, Stam C J, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res, 2006, 87: 60—66
74 Pachou E, Vourkas M, Simos P, et al. Working memory in schizophrenia: An EEG study using power spectrum and coherence analysis to estimate cortical activation and network behavior. Brain Topogr, 2008, 21: 128—137
75 Rubinov M, Knock S A, Stam C J, et al. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp, 2009, 30: 403—416
76 Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain, 2008, 131: 945—961
77 Wang L, Zhu C Z, He Y, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp, 2009, 30: 638—349
78 Ponten S C, Bartolomei F, Stam C J. Small-world networks and epilepsy: Graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol, 2007, 118: 918—927
79 Leistedt S J, Coumans N, Dumont M, et al. Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp, 2009, 30: 2207—2219
80 Collins D L, Holmes C J, Peters T M. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp, 1995, 3: 190—208 1582
81 Liang X, Wang J H, Yan CG, et al. Different correlation metrics reveal different topological patterns in the human brain functional net- works. NeuroImage, 2009, 47: S170
82 He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer’s disease: A combined structural and rest- ing-state functional MRI study. Neuroimage, 2007, 35: 488—500
83 Honey C J, Kotter R, Breakspear M, et al. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA, 2007, 104: 10240—10245
84 Greicius M D, Supekar K, Menon V, et al. Resting-state functional connectivity reflects structural connectivity in the default mode net- work. Cereb Cortex, 2009, 19: 72—78
85 Park C H, Kim S Y, Kim Y H, et al. Comparison of the small-world topology between anatomical and functional connectivity in the hu- man brain. Physica A, 2008, 387: 5958—5962
86 Honey C J, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA, 2009, 106: 2035—2040
不足/问题
1.如何构建符合脑工作机理的脑结构和脑功能网络
2.如何评价不同的节点定义方法对脑功能网络的影响并确定最合理的定义方法
3.如何评价不同的大脑网络连接定义方法对脑功能网络的影响并确定最合理的定义方法
4.需要构建邮箱的结构和功能网络来刻画不同脑区纤维连接的方向性及其神经活动之间的因果关系,以期更加深入细致的理解大脑的结构组织模式和功能活动规律
5.如何在更小的时间尺度上,如在每个时间点上构建动态的脑网络来了解大脑功能拓扑组织结构随时间变化的规律,从而进一步探索大脑实时的功能活动机制
6.探索从静息态到任务态,大脑功能网络的组织结构回发生怎样的变化
7.不同任职状态下,大脑功能网络的组织结构是否会表现出不同的局部调节方式
8.如何结合多模态成像技术全面定量的评价脑结构-功能网络的相似性和特异性,并理解结构网络组织模式对大脑功能形成的作用以及大脑功能的塑造对大脑结构的影响
9.虽然已有研究表明不同神经精神疾病下大脑结构和功能网络的拓扑结构会发生异常变化,但对于各种疾病下脑网络拓扑参数的变化趋势和幅度仍然没有统一的结论
10.如何融合多模态成像技术分析和理解神经精神疾病的病理生理机制,并建立可靠有效的影像学诊断标记
知识结构
细碎知识
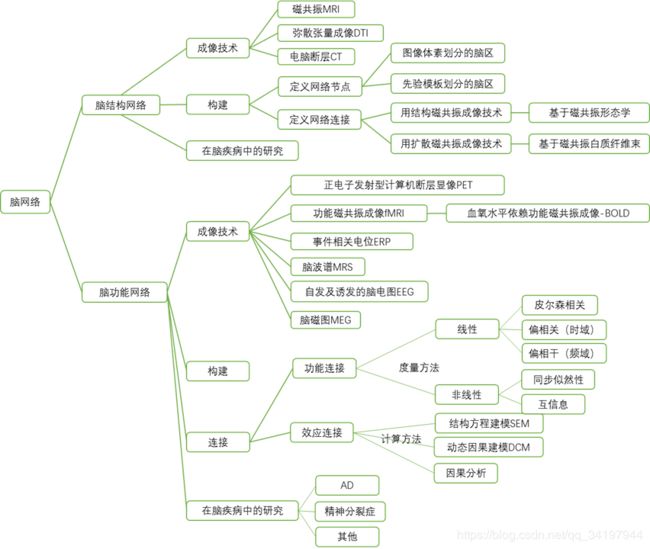
1.脑结构网络.:一个成年人的大脑中约有 1011 个神经元细胞, 这些数 量巨大的神经元细胞通过大约 1015 个突触互相连接, 形成了一个高度复杂的脑结构网络。
2.大脑功能网络:大脑结构 网络上动力学过程的同步化将大脑在广泛的时空尺度上连接形成了动态的复杂功能网络, 从而使人脑 连接组的研究从大脑结构网络扩展到了大脑功能网络
3.人脑连接组 3个空间尺 度,:微尺度(microscale)、中间尺度(mesoscale)和大尺度(macroscale 或 large-scale) (分别代表神经元、神经元集群和大脑脑区 3 个水平)
4.目前该领域的研究主要
- 集中在大尺度水平上
- 通过结构磁共振成像、扩散磁共振成像等成像技术来构建大脑结构连接网络或者采用脑电图、脑磁图和功能磁共振成像等技术建立大脑功能连接网络
- 结合基于图论(graph theory)的复杂网络分析方法,
- 揭示 其拓扑原理, 进而理解大脑内部的工作机制。
5.复杂网络的基本统计指标:节点度及其分布特征、集群系数、局部效率、最短路径长度、全局效率、中心度、模块、度相关、模体、等级性等。Boccaletti S, Latora V, Moreno Y, et al. Complex networks: Structure and dynamics. Phys Rep, 2006, 424:175—308
6.复杂网络的基本统计性质:最终的“小世界”属性和“无标度性”。
7.“小世界”网络兼具高集群系数和最短路径长度,Humphries等人将两个度量指标统一为一个标量![]() 来衡量。其中,
来衡量。其中,![]() ,
,![]() 近似于1(real表示真实网络,random表示随机网络)。当
近似于1(real表示真实网络,random表示随机网络)。当![]() >1时网络具有小世界属性,
>1时网络具有小世界属性,![]() 越大网络的小世界属性越强。在信息传递和处理的过程中具有相对较高的局部效率和全局效率。
越大网络的小世界属性越强。在信息传递和处理的过程中具有相对较高的局部效率和全局效率。
8.无标度网络。网络中节点与节点之间的连接分布遵从幂律分布P(k)~![]() ,该分布的形状通常在双对数坐标下近似一条直线。无标度网络中大部分节点只有少数连接,而有少数节点却拥有大量的连接,网络中节点重要性呈现出强烈的异质性,这种异质性使得无标度网络在面临意外攻击时表现出很强的鲁棒性,因为随机攻击的对象往往主要是网络中占大多数的只有少数连接的非核心节点,而这些非核心节点的损失不会对网络的拓扑结构产生重大的影响。但同时,若遇到针对核心节点的共计,表现出无标度网络脆弱的一面。无标度网络中节点的重要性具有极端的两极分化,表明网络中存在超级重要的核心节点,这些核心节点在维持整个网络的完整性和连通性中发挥着不可估量的作用。
,该分布的形状通常在双对数坐标下近似一条直线。无标度网络中大部分节点只有少数连接,而有少数节点却拥有大量的连接,网络中节点重要性呈现出强烈的异质性,这种异质性使得无标度网络在面临意外攻击时表现出很强的鲁棒性,因为随机攻击的对象往往主要是网络中占大多数的只有少数连接的非核心节点,而这些非核心节点的损失不会对网络的拓扑结构产生重大的影响。但同时,若遇到针对核心节点的共计,表现出无标度网络脆弱的一面。无标度网络中节点的重要性具有极端的两极分化,表明网络中存在超级重要的核心节点,这些核心节点在维持整个网络的完整性和连通性中发挥着不可估量的作用。
9.规则网络。具有较高的集群系数和较长的最短路径长度。
10.随机网络。具有较低的集群系数和较短的最短路径长度。
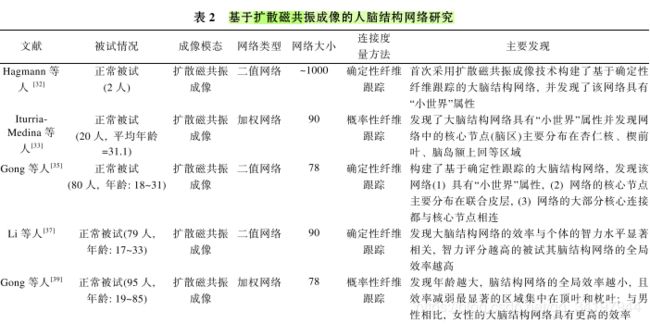
11.研究者发现结构磁共振成像和扩散磁共振成像能够获取活体人脑的结构连接信息,这使得构建人脑结构网络成为可能。
12.构建大脑结构网络关键的两步:一是如何定义网络节点;二是如何定义网络连接(边)。其中,节点的定义依赖于研究的空间尺度,一半在大尺度上,以图像体素/先验模板划分的脑区定义大脑结构网络的节点。而网络连接的定义则依赖于不同模态的成像技术,采用结构磁共振成像可以获得闹得形态学数据,比如灰质密度、灰质体积、以及皮层厚度等。脑结构网络的形态学连接就是根据脑区之间形态学数据的相关性来定义的;通过扩散磁共振成像可以检测到不同脑区之间的白质纤维束,大脑的结构连接(解剖连接)网络是利用白质纤维的连接数目、密度、强度、概率等定义的。
13.结构磁共振成像大量被用于研究人脑正常发育、老化和疾病过程中局部脑区形态学的变化,尽 管目前我们还不清楚脑区间形态学相关性确切的生 理意义, 但是一些研究已经表明这种形态特征的协 调变化可能与先天遗传[24]及后天的可塑性[25]有关
14.大脑功能网络是对大脑结构网络之上不同的神经元、神经元集群或脑区之间动态活动交互整合的直 观描述. 但与结构网络一样,
15.脑功能网络的连接指不同节点记录的神经 活动信号之间的动态协调性,这种协调性的定义主 要包括功能连接和效应连接(effective connectivity)两 种描述. 功能连接[45]是指空间上分离的神经单元其神经活动在时间上的关联性或统计依赖关系, 其主 要的度量方法分为线性和非线性两类[46], 线性方法 包括皮尔森相关、偏相关(时域)及偏相干(频域)等, 非线性方法主要有同步似然性(synchronization like- lihood, SL)、互信息等;
16.效应连接则刻画了一个神经单元对另一个神经单元神经活动的因果效应, 即二 者之间调控与被调控的关系, 该度量属于有向连接, 主要计算方法包括结构方程建模(structural equation modeling, SEM)、动态因果建模(dynamic causal modeling, DCM)和因果关系分析(granger causality)等.
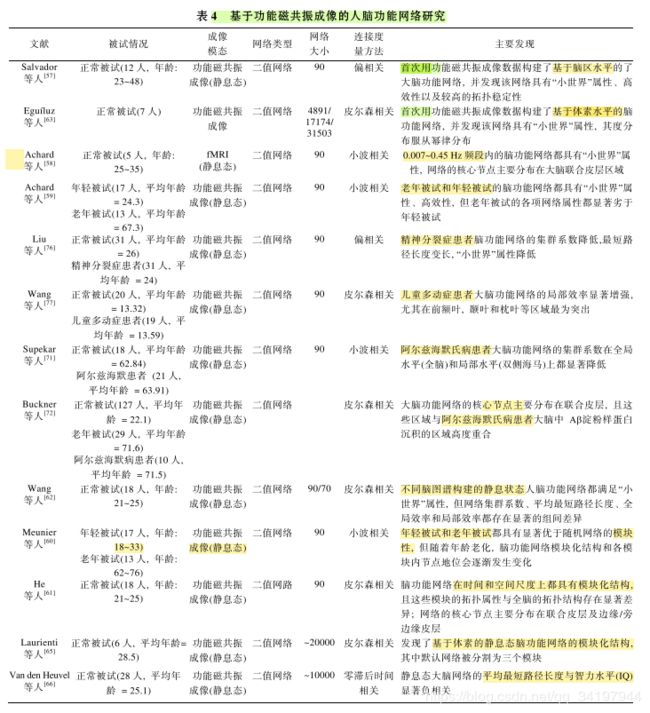
17.基于血液氧合水平(blood oxygenation level de-pendent, BOLD)的功能磁共振成像技术[54]因兼有较 高的时间分辨率和空间分辨率, 为研究人脑的功能 提供了一种重要的手段. 特别是静息态的 fMRI[55]由于能够反映人脑的自发神经活动, 已经成为神经科 学领域和神经精神疾病领域的研究热点.
18.静息态 fMRI 是指, 在无特定任务的情况下, 受试者在不做 系统地思考或尽量不要思考问题的状态下进行的磁 共振扫描. 研究表明,
19.静息态 fMRI 信号的低频振 荡与自发的神经元活动关系密切, 具有比较明确的生理意义与病理意义
20.因此基于体素水平的脑功能网络研究可能 会提供新的网络拓扑信息和结果.
21.默认网络:默认状态网络(default state network),任务负网络(task-negative network: TNN)。https://blog.csdn.net/qq_34197944/article/details/102911207
22.其他概念https://blog.csdn.net/qq_34197944/article/details/102910395